Layered oxide cathodes for potassium-ion batteries: challenges, strategies and perspectives
Abstract
Potassium-ion batteries are currently garnering extensive focus on account of the cost-effectiveness and generous supply of potassium resources. Investigating outstanding electrode materials that exhibit favorable performance is essential for the advancement of these batteries. Layered transition metal oxides have emerged as a highly promising cathode material owing to their high capacity and facile synthesis. However, their effective application is hindered due to inadequate performance, which can be ascribed to irreversible phase transition, air instability and interfacial instability. Herein, this review comprehensively outlines the causes of these three key scientific issues and proposes some corresponding optimization strategies, mainly including element doping, surface modification, structural design, and electrolyte optimization, with the aim of offering insights for the prospective advancement of potassium-based layered oxide cathode materials.
Keywords
INTRODUCTION
Renewable energy sources such as wind, solar and wave power have recently gained increased attention because of the significant pollution caused by the extensive use of fossil fuels[1,2]. However, the irregularity and fluctuations of these resources impede their direct integration into the grid, resulting in serious resource wastage. Therefore, the establishment of large-scale electrical energy storage (EES) systems is imperative[3]. Lithium-ion batteries (LIBs) have been extensively utilized in various fields over the recent decades, thanks to their high energy density, superior conversion efficiency, low self-discharge rate and long cycling life[4]. However, the lithium reserves are restricted (merely 0.0017 wt% of Earth’s crust) and distributed disparately across its expanse, causing a sustained increase in the price of Li2CO3 which may further restrict its application in EES and the electric vehicle market[5]. Compared with lithium, potassium has a relatively high natural abundance (2.47 wt% of Earth’s crust) and exhibits similar physical and chemical properties to lithium, establishing potassium-ion batteries (PIBs) as a promising alternative to LIBs. Besides, PIBs have a working principle similar to LIBs by shuttling the K+ between the cathode and anode[6-8]. Recently, research on PIBs has received significant interest because of their distinctive properties, which set them apart from both LIBs and sodium-ion batteries (SIBs): (1) graphite can be served as the anode in PIBs, whereas it is not effective for SIBs because Na+ ions cannot be intercalated into graphite[9]; (2) K+ has a smaller Stokes radius, means it exhibits the lowest de/solvation energies among alkali metal ions. The mild interaction of K+ ions with solvent molecules allows for faster ion transfer and higher ion conductivity, which can stimulate the potential in low-temperature performance[10,11]; (3) The standard potential for K+/K, standing at -2.93 V concerning a standard hydrogen electrode, is lower than that of
The electrode materials are crucial in determining electrochemical properties. Anode materials suitable for PIBs can be mainly classified into carbon-based materials (such as graphite, soft carbon and hard carbon), alloys, organic materials and conversion-type anode[14,15]. Among them, graphite is the most promising anode for PIBs due to its high theoretical capacity (279 mA h g-1) and low cost. However, a large volume variation during potassiation/depotassiation would irreversibly damage the solid electrolyte interface layers, thereby resulting in poor rate performances and cycling stability. In recent years, researchers have made significant improvements in their electrochemical properties by extending the interlayer space of graphite and constructing a coating layer. The incorporation of S/N heteroatoms into graphite nanosheets yielded an expanded interlayer spacing of 0.39 nm, coupled with a high density of structural defects and electroactive sites, which synergistically enabled exceptional cycling stability, retaining a capacity of 142 mA h g-1 even after 5,000 cycles at a current density of 0.5 A g-1[16]. In sharp contrast to recent progress in anode materials, the low capacity and short cycle life of the current potassium cathode materials fall short of commercial energy density targets, which severely limits the viability of PIBs for grid-scale energy storage. The cathode materials of PIBs mainly include layered transition metal oxides, Prussian blue analogs (PBAs), polyanionic frameworks and organic compounds[17-20]. PBAs, characterized by their open framework structure, exhibit high capacity and high voltage but suffer from structural instability due to crystal water-induced collapse and low electronic conductivity. Organic cathodes offer high theoretical capacities through multi-electron redox reactions, yet their practical application is hindered by severe dissolution in electrolytes and low operating voltages. Polyanionic compounds leverage robust covalent frameworks to deliver exceptional cyclability and high voltage, though their low electronic/ionic conductivity result in sluggish kinetics causing poor rate performance in PIBs. Motivated by the superior electrochemical performance of layered transition metal oxides in SIBs[21], layered oxides have also become a research hotspot for PIBs. Compared to other cathode materials, potassium layered oxides exhibit two-dimensional diffusion channels that furnish abundant active sites and expanded spatial accommodation for K+ (de)intercalation during electrochemical cycling, which contributes to enhanced specific capacity. Furthermore, the incorporation of high redox potential elements such as nickel and cobalt enables the cathode to achieve high working voltage, synergistically enhancing energy density. Additionally, their compatibility with mature synthesis techniques and low raw material costs render them favorable for scalable production[22,23]. However, critical challenges also exist. During the K+ ion insertion and extraction from the cathode, the large radius of K+ ions leads to severe phase transitions, resulting in poor electrochemical performance[24]. Besides, the layered oxide cathodes of PIBs generally experience severe structural degradation when exposed to ambient conditions, impairing cell performance and presenting a substantial challenge for material storage[25]. Furthermore, the Coulombic efficiency (CE) and cycle life are often unsatisfactory in PIBs, which may be related to severe side reactions occurring between the electrode and the electrolyte[26]. Additionally, the interphase between the cathode and electrolyte is also an important part of ensuring long life cycling[27]. Interfacial stability is essential for achieving better electrochemical performance.
To date, many approaches have been proposed for the modification of K layered oxides; however, there is currently a limited comprehensive understanding regarding the relationship between degradation mechanisms and optimization strategies. This review addresses these gaps by establishing structure-property-performance relationships from cathode bulk to interphase dynamics, thereby unifying fragmented knowledge into actionable design principles. In this review, the mechanism of the main problems, including irreversible phase transition, air instability and interfacial instability, are discussed. In addition, optimization strategies including element doping, surface modification, structure design and electrolyte optimization are summarized to enhance the electrochemical performance of PIBs. By bridging fundamental degradation mechanisms with scalable engineering solutions, this review provides a roadmap for accelerating PIBs toward commercial viability in EES applications.
STRUCTURE CLASSIFICATION
Layer transition metal oxides typically are composed of edge-shared TMO6 octahedral layers and alkali layers, which can be given using a simple expression such as AxTMO2 (0 < x < 1, where A represents alkali metals such as Li, Na, K, etc., and TM indicates transition metal such as Ni, Fe, Mn, etc.). Different phases will be formed when TMO2 layers stack along the c-axis in different ways. Generally, the K layered oxides can be identified as P-type (P2, P3) and O-type (O3), where P denotes K+ ions in prismatic sites and O denotes K+ ions occupying octahedral sites, respectively, while number 2 or 3 indicates the number of TMO2 layers in distinct stacking modes, as shown in Figure 1. In addition, in instances where the structure exhibits slight distortion, a prime symbol (') is incorporated to differentiate it from the original structure[28-30].
P2-type layered oxides for PIBs are usually regarded as 2H phases with P63/mmc space groups, and the K+ ions are accommodated at prismatic sites with two types of TMO2 layers: AB and BA[31]. There are two different sites for K ions in an alkali layer: Ke and Kf, which represent edge-shared and face-shared TMO6 octahedral, respectively. Because of the large K+ ion radius and strong coulombic repulsion, the two kinds of K sites usually cannot be occupied at the same time. K+ ions migrate through 2D prismatic channels accompanied by redox reactions of transition metals during charging and discharging process. Typically, during deep potassium extraction, the P2 phase will transform to O2 phase, which has an AB-AC oxygen stacking sequence, caused by the increased interlayer repulsion of TMO2 layers. The compressed interlayer spacing and high stress will be generated, accompanied by phase transitions, which greatly influence the specific capacity and cycling life of the cathode[32].
For P3-type oxides, one of the triangular faces of each KO6 prism is shared with a TMO6 octahedron, and the remaining triangular face shares its edges with three distinct TMO6 octahedra on the opposing side. Differing from the P2 phase, the oxygen stacking sequence in the P3 phase follows the AB-BC-CA arrangement along the c-axis with the R3m space group[33]. In addition, the P3 phase is usually obtained at a low synthesis temperature, while the P2 phase is typically achieved at a high synthesis temperature, which suggests that it is difficult for the P3 phase to transform to the P2 phase during charging-discharging process without breaking TM-O band because of the low energy. In P3-type oxides, the prismatic sites provide more flexible diffusion channels, enabling a smoother K+ migration with reduced energy barriers. The P3 phase usually transforms to the O3 phase under high voltage operation, caused by the gliding of TMO6 octahedral slabs by (1/3, 2/3, 0) without breaking TM-O bands[34]. P-type layered oxides offer high ionic conductivity and moderate capacity but suffer from irreversible phase transitions and interface instability. Structural engineering (e.g., doping, coating) and optimized electrolyte formulations are critical for enhancing their cyclability which will be discussed in detail later.
O3-type layered oxides are usually designed as a 3R phase with R
In PIBs, P-type layered oxide cathodes are more commonly synthesized compared to O-type layered oxides. This preference is linked to the larger ionic radius of K+ ions, which results in strong electrostatic interactions between ions when they occupy octahedral sites, thereby destabilizing the layered structure[36]. Among various P-type cathodes, manganese-based layered oxides have emerged as a prominent research focus due to the plentiful supply and low cost of manganese resources. Liu et al. synthesize both P2-type and P3-type KxMnO2 with potassium contents of 0.3 and 0.45, respectively, which indicates that the potassium content is a crucial factor influencing the formation of different phases[37]. Moreover, Co-based and Cr-based oxides serve as popular model systems[38,39]. However, only KSrO2 and KCrO2 have been identified to currently exhibit a thermodynamically stable O3 phase, which was unveiled through the utilization of Density Functional Theory (DFT) to compute the energy above hull for different O3-layered KTMO2 compounds[40]. Single-transition metal oxides frequently demonstrate multiple phase transitions and limited structural stability when extracting K+ ions from the alkali ion layer. Harnessing the synergistic effects of diverse elements usually leads to enhanced performance in cathode materials.
SYNTHESIS METHOD
The development of high-performance layered oxide cathodes for PIBs relies heavily on advanced synthesis techniques to achieve structural stability, phase purity, and optimized K+ diffusion kinetics. The synthesis procedures for K layered oxides primarily encompass solid-state reactions, sol-gel processes, co-precipitation synthesis, hydrothermal/solvothermal techniques and electrochemical ion exchange. Their respective merits and drawbacks will be critically evaluated in subsequent sections.
The solid-state reaction is the most commonly used method for the preparation of layered oxides. It obtains the product by mixing potassium salts and oxide precursors, pressing them into pellets, and then sintering at high temperatures. The method is facile, but it is difficult to regulate the particle size and morphology. Scanning electron microscopy (SEM) characterization revealed that the as-prepared P3-K0.5MnO2 oxide exhibits plate-like morphology with particle sizes ranging from 1 to 2 μm[41]. This morphology typically compromises electrochemical performance due to limited interparticle contact areas and insufficient ionic diffusion pathways. Furthermore, the conventional solid-state method fails to ensure atomic-scale homogeneity, leading to detectable impurity phases. A series of K0.67Mn1-xNixO2
Sol-gel method involves dissolving metal precursors (such as nitrates or acetates) in a solvent, followed by chelation with organic agents (such as citric acid, ethylene glycol) to form a homogeneous sol. Subsequently, the sol transitions into a gel, which is then dried and calcined at high temperatures to yield target oxides. The sol-gel-derived K0.7Mn0.7Ni0.3O2 exhibits phase-pure P3-type structure without detectable NiO impurities and superior performance (124.2 mA h g-1 at 0.1 A g-1, a capacity retention of 86.2% at 0.5 A g-1 after 500 cycles)[43]. However, sol-gel synthesis is complex, requiring meticulous optimization of pH, temperature, and chelating agent ratios. The method is time-intensive, prone to organic residue contamination, and less scalable compared to solid-state, limiting its industrial applicability. Despite these drawbacks, its capacity to produce high-performance cathodes makes it a preferred choice for lab-scale PIB research.
Co-precipitation synthesis is a solution-based method for preparing layered oxide cathodes for PIBs. This technique involves dissolving transition metal salts (such as sulfates or nitrates) in aqueous solutions, followed by the addition of a precipitating agent (such as NaOH or KOH) under controlled pH and temperature to form homogeneous hydroxides or carbonates. The precipitates are filtered, dried, and calcined with a potassium source to yield layered oxides. Co-precipitation enables uniform particle size distribution and minimizes cation mixing. Peng et al. developed hollow K0.5MnO2 submicrospheres via co-precipitation method[44]. SEM imaging reveals well-defined spherical morphology with uniform particle sizes averaging 600 nm in diameter. Thanks to the special structure, the cathode achieves a capacity retention of 89.1% at 200 mA g-1 after 400 cycles. It is worth noting that the precipitation agent, pH, stirring speed, and temperature must be carefully adjusted to ensure the quality of the products.
Hydrothermal/Solvothermal Synthesis is also a solution-based technique for fabricating K layered oxide cathodes where precursors react in a sealed autoclave under elevated temperatures and pressures, using water (hydrothermal) or organic solvents (solvothermal) as reaction media. This method enables precise control of nanostructured morphologies, such as nanowires or microspheres, which enhance K+ diffusion kinetics. Deng et al. synthesized K0.65Fe0.5Mn0.5O2 cathodes by two different routes (hydrothermal and conventional solid-state reactions) to demonstrate the influence of the synthetic procedure on the properties of the cathode[45]. The s-K0.65Fe0.5Mn0.5O2 microsphere was synthesized via hydrothermal method. Conversely, the c-K0.65Fe0.5Mn0.5O2 was synthesized through the solid-state route. The results show that the
Electrochemical ion exchange is an advanced method to prepare layered oxide cathodes for PIBs by leveraging controlled voltage or current to drive the exchange of pre-inserted ions (e.g., Na+) with K+ ions in an electrolyte medium. This method leverages the structural stability of sodium-based oxides to create stable potassium phases that are challenging to synthesize directly. The Na0.67Li0.12Ni0.22Mn0.66O2 cathode was galvanostatically cycled in a cell with the configuration K-metal|KClO4 in ethylene carbonate (EC):diethyl carbonate (DEC)|Na0.67Li0.12Ni0.22Mn0.66O2[46]. The Na+ ions are gradually substituted with K+ ions, yielding P2-
CHALLENGES
While K layered oxides exhibit a multitude of advantageous characteristics, their practical application is constrained by suboptimal electrochemical performance, influenced by a combination of factors[48,49]. Throughout the continuous charge and discharge cycles, the extraction/insertion of larger K+ ions typically induces volume contraction/expansion, potentially leading to irreversible phase transitions and hastening electrode degradation. Additionally, exposure to air frequently results in a significant decline in cathode performance, largely due to surface reconstruction and K+/H+ ion exchange. Interfaces, serving as the primary sites for charge transfer, have an important impact on ion transport and cycling stability. Herein, the fundamental reaction mechanisms underlying these challenges and their association with electrochemical performance will be thoroughly discussed below.
Irreversible phase transition
Irreversible phase transitions commonly occur at high voltage regions because of the alterations in interlayer electrostatic forces during the de/intercalation of K+ ions. Some phase transitions can have a beneficial impact on layered oxides. For instance, the transformation from O-type to P-type can enhance the diffusion of K+ ions by bypassing the higher diffusion barrier in tetrahedral sites[50]. Conversely, irreversible phase transitions are responsible for the decline in electrochemical performance of K layered oxides. Besides, the persistent effects of interfacial stress often lead to the formation of intragranular cracks, which enlarge the contact surface between electrolyte and electrodes, exacerbating side reactions and reinforcing the fatigue of layered oxides[51].
Layered P3-K0.5MnO2 exhibits a high capacity of 140 mA h g-1 within the voltage range of 1.5-4.2 V, characterized by two striking plateau features at 3.7 and 4.1 V (vs. K+/K) during the K+ extraction. However, these voltage plateaus become less prominent during the subsequent discharge process [Figure 2A][52]. Subsequently, a P3-O3 phase transition was revealed by in-situ XRD and DFT calculation upon K+ extraction to 0.41 which is primarily driven by K+/vacancy ordering and sliding of TMO2 layers [Figure 2B]. The results suggest that the P3-type structure demonstrates minimal energy levels with increased K content, while the O3-type framework achieves stability with reduced potassium content. Notably, with further extraction of K+ ions, a special phase, defined as the X phase, appears, characterized by the (104) peak in the O3 phase and additional characteristic peaks at the left of the (003) and (006) peaks. Unlike P-type cathodes, the O-type cathodes undergo more intricate phase transitions during de/potassiation. O3-KCrO2 delivers an initial discharge capacity of 92 mA h g-1 and retains a capacity of 70 mA h g-1 after 20 cycles at 5 mA g-1 accompanying multiple voltage plateaus, which may relate to the complex phase transitions when the content of potassium changes [Figure 2C][40]. The structural evolution, as revealed by in-situ XRD, shows a complex series of phase transitions (O3-O'3-P'3-P3-P'3-P3-O3) during the charging process [Figure 2D]. However, the cathode does not revert to its initial O3 phase after discharge, a key factor contributing to its poor cycling stability. The intensity of phase transitions observed in KxCrO2 is plausibly related to the interaction between K+ ions, necessitating the formation of multiple intermediary phases to mitigate such interactions with increasing potassium content. Interestingly, the authors noted that before the in-situ experiments, phase separation occurred within the electrode, attributed to partial K+ ion deintercalation after mixing KCrO2 with carbon.
Figure 2. (A) GCD curves and (B) In-situ XRD pattern of P3-K0.5MnO2 between 1.5-4.2 V[52]. (A and B) Reprinted with permission from Ref.[52]. Copyright 2017 Wiley-VCH[52]. (C) GCD curves and (D) In-situ XRD pattern of O3-KCrO2[40]. (C and D) Figures reprinted with permission from Ref.[40]. Copyright 2018 American Chemical Society[40].
Air instability
The two-dimensional diffusion pathway in layered oxides is conducive to ion transport; nonetheless, atmospheric molecules such as water and CO2 can readily infiltrate, triggering detrimental side reactions[53]. Firstly, carbon dioxide and water molecules adsorbed on the particle surfaces react with the deintercalated alkali metal ions, leading to the generation of surface residual alkali compounds (carbonates, hydroxides,
The mechanisms responsible for the poor air stability of layered oxides represent a long-standing topic of contention within scientific discourse[57-59]. Recently, Yang et al. conducted a systematic investigation into the impact of various atmospheres (water vapor, oxygen, carbon dioxide) on the degradation of Na layered oxides, using O3-NaNi1/3Fe1/3Mn1/3O2 (NFM111) as the model system[60]. Researchers discovered that the XRD pattern maintained its original characteristics without any new peaks even after 48 h of individual exposure to water vapor, oxygen and carbon dioxide, or simultaneous exposure to oxygen and carbon dioxide for 48 h. However, peak shifts were observed in the presence of both water and oxygen. Further, in the combined presence of water and carbon dioxide, the formation of sodium bicarbonate occurred, highlighting water as a primary factor contributing to performance degradation [Figure 3A]. The rough surface and increased spacing of lattice fringes further prove the damaging effect of combined water vapor and CO2. The intercalation of CO2, CO32-, H3O+, or H2O is ruled out by conducting Time-of-Flight Secondary Ion Mass Spectrometry (TOF-SIMS) analyses and isotope-labeling strategies [Figure 3B]. Furthermore, powder neutron diffraction confirmed the integration of protons into tetrahedral sites within the sodium layers, potentially triggering acid degradation [Figure 3C]. Subsequent X-ray Absorption Spectroscopy (XAS) analysis demonstrated the oxidation of bulk Ni2+ by O2, a phenomenon that becomes more pronounced with higher Ni content, indicating the inadequate air stability of high-nickel materials [Figure 3D].
Figure 3. (A) XRD patterns of NFM111 exposed to different atmospheres with magnified regions of (003), (104), and (110)[60]. (B) 3D rendering of selected representative secondary-ion fragments from ToF-SIMS characterization. Samples were stored in N2 with H218O vapor for 72 h and in CO2 with H218O vapor for 24 h[60]. (C) Refinement of the NPD pattern of the sample stored in D2O vapor and CO2 for 12 h, where D atoms are placed at the tetrahedral sites in the Na layer (Natetra in inset)[60]. (D) Ni K-edge XAS spectra of NFM111 and NFM424 before and after being stored in O2 with water vapor (RH = 80%) for 48 h[60]. (A-D) Figures reprinted with permission from Ref.[60]. Copyright 2024 The American Association for the Advancement of Science[60]. (E) The XRD patterns of KFMTO and KFMTO·0.16H2O powder being aged in the air for seven days[61]. Reprinted with permission from Ref.[61]. Copyright 2022 Wiley-VCH[61]. (F) cyclic performance of KMO and exp-KMO at 0.5 C[62]. Reprinted with permission from Ref.[62]. Copyright 2024 Wiley-VCH[62].
Owing to the larger radius of K+ ions, the inferior air stability is notably pronounced in K layered oxides. P3-K0.4Fe0.1Mn0.8Ti0.1O2 delivers a capacity of 115 mA h g-1 at 20 mA g-1 based on Fe3+/Fe4+ and Mn3+/Mn4+ redox couple[61]. However, significant capacity degradation was observed following a 7-day storage period in air, causing only 76 mA h g-1 to remain. XRD patterns reveal new diffraction peaks corresponding to K2CO3 and MnO phases [Figure 3E], which may originate from the K+/H+ exchange and disproportionation reaction of Mn3+, respectively. The air instability of K layered oxides is a widely acknowledged issue. The XRD Rietveld refinement of P3-K0.5Mn0.7Fe0.2Mg0.05Cu0.05O2 (KMO) revealed the appearance of diffraction peaks, signaling the presence of H2O and carbonate species after 14 days of exposure to air[62]. And the surface of particles displayed increased imperfections post-exposure, adversely impacting ion transport kinetics. As depicted in Figure 3F, the discharge capacity of the exposed cathode (exp-KMO) only reached 46.1 mA h g-1 after 100 cycles at 0.5 C, representing 57.3% of the capacity exhibited by the fresh sample.
In short, the degradation process of the cathode in air can be divided into two stages [Figure 4]: (1) acid degradation. The layered oxides experience Na+/H+ ion exchange in air causing the removal of Na+ ions and surface reduction of Mn4+ or Mn3+, which results in significant cracking, curvature within interlayers, and surface reconstruction; (2) oxidative degradation. The transition metal ions are oxidized by water and oxygen, prompting the deintercalation of sodium ions and the formation of harmful residues on the surface, yielding the formation of a rock salt phase at the surface and a decrease in performance.
Interfacial instability
When an electrode interacts with an electrolyte, or throughout the procedures of charging and discharging, the formation of an electrode-electrolyte interphase (EEI) occurs on the electrode surfaces as a consequence of electrolyte reduction or oxidation, resulting from the thermodynamic equilibrium of energy levels between the electrodes and electrolyte [Figure 5A][63]. While the electrochemical potential of the cathode is below the highest occupied molecular orbital (HOMO) energy level of electrolyte, electrons transfer from the electrolyte to the cathode, causing electrolyte oxidation. In contrast, with the electrochemical potential of the anode surpassing the lowest unoccupied molecular orbital (LUMO) energy level of the electrolyte, electrons move from the negative electrode to the electrolyte, leading to electrolyte reduction. The EEI between the cathode and electrolyte is usually called a cathode electrolyte interphase (CEI). The interphase on the anode side is referred to as a solid electrolyte interphase (SEI)[64,65].
Figure 5. (A) Schematic illustrating the energy states of the electrodes and the electrolyte, leading to the formation of the SEI and cathode electrolyte interphase[63]. Reprinted with permission from Ref.[63]. Copyright 2020 Royal Society of Chemistry[63]. (B) The ligand field in manganese oxides and the schematic diagram of the J-T distortion of Mn3+[70]. (C) Diagram of transition metal ion dissolution[70]. (B and C) Figures reprinted with permission from Ref.[70]. Copyright 2020 The American Association for the Advancement of Science[70]. (D) The Fe 2p and Mn 2p spectra from the light-yellow region of the separator after being cycled in cells 20 times at 25 and 45 °C[71]. (E) EELS spectra of O K-edge, Mn, Co and Ni L-edges collected from the surface to the subsurface on a representative particle with an increment of 1 nm per spectrum[71]. (D and E) Figures reprinted with permission from Ref.[71]. Copyright 2018 Wiley-VCH[71]. (F) CV curves at the scan rate of 0.1 mV s-1 for KNMO in the initial five cycles[74]. (G) SEM image of the cycled electrodes[74]. (F and G) Figures reprinted with permission from Ref.[74]. Copyright 2023 Wiley-VCH[74].
The EEI typically acts as an ion conductor and electron insulator to ensure the stable cycling of batteries. On the cathode side, a robust and stable CEI is of critical importance in safeguarding the performance of the cathode material[66,67]. Firstly, the occurrence of electron tunneling will continuously deplete the electrolyte if the cathode is not effectively passivated, leading to a decline in electrochemical performance. The CEI film formed on the surface of NFM111 in 1 M NaPF6 in propylene carbonate (PC)/ethyl methyl carbonate (EMC) with 2% fluoroethylene carbonate (FEC) exhibits suboptimal thickness (1 nm), which is inadequate for efficient passivation of the electrode, accelerating the dissolution of transition metal ions[68]. Secondly, the electrolyte, which contains PF6- ions or FEC, has the potential to generate HF during decomposition when subjected to high voltage[69]. Additionally, the unequal distribution of electrons within the eg orbitals of Jahn-Teller effect ions (e.g., Mn3+, Cu2+, Fe4+) may result in varying degrees of stretching or compression in the TM-O bonds of the TMO6 octahedra [Figure 5B]. It has been demonstrated that oxygen atoms on elongated TM-O bonds possess a more negative charge, rendering them susceptible to reacting with HF. Consequently, transition metal ions may subsequently dissolve into the electrolyte, causing structural collapse [Figure 5C][70]. The X-ray Photoelectron Spectroscopy (XPS) spectra on post-cycled separators further confirmed the dissolution of iron and manganese [Figure 5D][71]. The results from electron energy loss spectroscopy (EELS) of O3-Na1/3Ni1/3Fe1/3Mn1/3O2 illustrate a low-to-high energy shift in the Mn L-edge from the surface to the bulk and an increasing intensity of the O K-edge which imply surface reduction of Mn and oxygen loss [Figure 5E]. Furthermore, the surface reconstruction, nano-cracks and dislocations arise from the side reactions between the electrode and electrolyte, diminishing the kinetic performance and reducing the cycling lifespan.
During the discharging process, the valence state of Mn undergoes a change from Mn4+ to Mn3+, which is followed by disproportionation reactions that transform Mn3+ into Mn2+ and Mn4+. Subsequently, the Mn2+ is dissolved into the electrolyte[22,72]. P3-K0.5MnO2 shows a notable specific capacity above 110 mA h g-1 at
STRATEGIES
As previously discussed, the bulk properties and interface characteristics of the cathode profoundly influence electrochemical performance. These attributes are primarily governed by three key factors: the composition of the cathode, the distribution of elements within the cathode, and the compatibility between the electrode and electrolyte. With this respect, numerous optimization strategies, such as element doping, surface modification, structure design and electrolyte optimization, have been demonstrated to significantly enhance electrode performance. Consequently, a detailed discussion of these strategies will be provided.
Element doping
KxMnO2, benefiting from the low cost of Mn resources and its outstanding capacity, has become a widely utilized K layered oxide cathode. However, the insufficient structural reversibility under high-voltage operation and transition metal dissolution during cycling have a detrimental impact on its performance[75-78]. It is recognized that the composition of the cathode is a key factor in determining the effect of the electrode. With the appropriate substitution of elements, the intrinsic crystal structure and ion state can be substantially modulated which can help to improve stability and suppress structure collapse.
Suppressing irreversible phase transition
The KxMnO2 cathode exhibits a complex structural transition during the charging and discharging process, resulting in a significant volume change and rapid capacity loss. To inhibit the P3-O3 phase transition and Jahn-Teller effect of Mn3+, the doping/substitution of the cathode with electrochemically active elements (Ni, Fe, Co, Cu, etc.) or inactive elements (Mg, Ti, Al, Zn, etc.) has been widely investigated.
Doping electrochemically active elements can both stabilize the structure and boost the capacity. Ni-substituted P2-K0.44Ni0.22Mn0.78O2 delivers a first discharge capacity of 125.5 mA h g-1 at 10 mA g-1 with a capacity retention of 67% after 500 cycles at 200 mA g-1 between 1.5-4 V based on Ni2+/Ni3+, Ni3+/Ni4+ and Mn3+/Mn4+ redox couple [Figure 6A][41]. Results from in-situ XRD found that there are no impurity peaks throughout the charging/discharging process indicating a single solid-solution reaction with small volume change of 1.5%. Besides nickel[42,79], other elements such as iron[80,81], nickel and cobalt[82-85] are also effective substitution elements to improve cycling life and electrical conductivity. P3-K0.5Mn0.8Fe0.1Ni0.1O2 exhibits a high specific capacity, coupled with a P3-O3 phase transition when charging from 3.5 to 3.9 V[79]. However, the O3-X phase transition observed in K0.5MnO2 disappears, which is attributed to the substitution of Mn3+ site by Fe3+ and Ni2+ suppressing the additional phase transition [Figure 7A]. In-situ XRD patterns of the P3-KxCo0.5Mn0.5O2 and DFT calculation confirmed a single-phase reaction during K+ extraction and insertion[82]. In the pristine P3-K0.75MnO2, two Mn-O bonds have longer stretches than the other bonds within the MnO6 octahedra. Conversely, all of the Mn-O bonds within the MnO6 octahedra exhibit similar lengths in the Co-substituted P3-K0.75Co0.5Mn0.5O2, indicating the effectiveness of Co-substitution in alleviating the Jahn-Teller distortion caused by Mn3+ in the cathode [Figure 7B]. Nevertheless, the widespread application of cobalt-containing layered oxides is constrained by the toxicity and high cost associated with cobalt[83].
Figure 6. GCD curves of (A) P2- K0.44Ni0.22Mn0.78O2[41]. Reprinted with permission from Ref.[41]. Copyright 2019 Wiley-VCH[41]. (B)
Figure 7. In-situ XRD patterns of (A) P3-K0.5Mn0.8Fe0.1Ni0.1O2[79]. Reprinted with permission from Ref[79]. Copyright 2020 Elsevier[79].
As electrochemically inactive ions do not engage in redox reactions, more K+ ions are maintained within the alkali metal layer, thus partially restraining the gilding of transition metal layers[86-89]. Huang et al. prepared a P3-K0.54Mn0.78Mg0.22O2 cathode, which exhibits a superior specific capacity of 132.4 mA h g-1 at
The implementation of light-weight ions (such as Li+[46,93,94], F-[95-97] and B3+[98,99]) within the transition metal layer facilitates the reinforcement of covalent bonds with adjacent O atoms and adjusts the redox behavior. P2-K0.56Na0.11Li0.12Ni0.22Mn0.66O2 obtained by electrochemical ion exchange exhibits a high reversible capacity and excellent structural stability at high voltage with a single solution reaction during the charging-discharging process [Figure 6D][46]. The Li-doped material displays a more even and continuous GCD curve than the pure sample, indicating the K+/vacancy ordering has been effectively mitigated. The F-doped P2-K2/3Mn7/9Ni1/9Ti1/9O17/9F1/9 cathode not only delivers a substantial capacity of 132.5 mA h g-1 within 1.5-4.2 V
In addition to substituting atoms within the transition metal layers, the introduction of inactive elements into the alkali metal layers to serve as interlayer pillars has been noted to impede the slippage of transition metal layers, consequently inhibiting phase transition[100-104]. Jo et al. have obtained
A high-entropy strategy with five or even more metal elements has been widely used in SIBs[105]. Electrochemical performance and material stability can be enhanced through the synergistic effects of multiple elements[106-110]. The ordering structure usually increases the diffusion barrier for K+ ions, thus inhibiting the diffusion dynamics. Conversely, the introduction of disorder not only facilitates the ion diffusion, improving the rate performance, but also generates additional storage sites for K+ ions. Analysis of Selected Area Electron Diffraction (SAED) patterns for K0.7Fe0.05Co0.1Mn0.75Ni0.05V0.05O2 (KFCMNV) revealed no evidence of superlattice reflections along the [-111] zone axis, in contrast to the ordered P3-KFCMN and P3-KFCMV[106]. The difference in redox couple potentials between P3-KFCMN and P3-KFCMV (0.413 and 0.526 V) was greater than that observed in P3-KFCMNV (0.386 V), indicating that the cathode with TM disordering exhibits commendable redox reversibility and superior rate performance. Besides inhibiting K+/vacancy ordering through element doping, Xiao et al. discovered that a K+/vacancy disordered structure can also be constructed by adjusting the potassium content[43]. Compared with K0.4Mn0.7Ni0.3O2, the superlattice spots are not visible in K0.7Mn0.7Ni0.3O2 through the SAED pattern. And the smoother GCD curves and CV curves additionally suggest a transition in the cathode structure from K+/vacancy ordering to K+/vacancy disordering with increasing potassium content [Figure 6G and H].
Enhancing air stability and interface stability
The aforementioned substituted elements primarily target the suppression of phase transitions and enhancement of structural stability. Additionally, certain metal ions show a positive impact on enhancing both air stability and interface stability[96,98,111,112]. Cu2+ has been shown to exhibit electrochemical activity in layered transition metal oxides for SIBs and can also enhance the air stability of layered cathodes effectively[113]. Park et al. combined machine learning and DFT calculations to design a P'3-K0.3Mn0.9Cu0.1O2 (KMCO) cathode[111]. The similar Mn-O band length in KMCO suggests a suppression of Jahn-Teller distortion. Significantly, analysis of the ex-situ XPS and ex-situ X-ray absorption near edge structure (XANES) spectra revealed a Cu2+/Cu3+ redox activity centered around 3.4 V, implying the electrochemical activity of Cu2+ in PIBs [Figure 8A]. Furthermore, the XRD patterns of KCMO displayed no discernible variance between the pristine samples and those exposed to air for four weeks, indicating a substantial enhancement in air stability associated with Cu2+ doping. In contrast, the presence of the hydrate phase and K2CO3 phase was evident in K0.3MnO2 following exposure to air [Figure 8B and C]. Al-doped
Figure 8. (A) Cu K-edge ex-situ XANES spectra of KMCO[111]. Comparison of XRD patterns of (B) K0.3MnO2 and (C) KMCO between pristine and after four-week air exposure[111]. (A-C) Figures reprinted with permission from Ref.[111]. Copyright 2021 Royal Society of Chemistry[111]. (D) HAADF-STEM image of K0.45Mn0.9Al0.1O2 along the [100] zone axes[112]. (E) Schematic illustration of the interphase layer for K0.45Mn0.9Al0.1O2 electrode after cycling[112]. (D and E) Figures reprinted with permission from Ref.[112]. Copyright 2023 Elsevier. (F) The XRD patterns of KFMTO and KFMTO·0.16H2O powder being aged in the air for seven days[61]. (G) GCD curves of the pristine and aged KFMTO and KFMTO·0.16H2O[61]. (F and G) Figures reprinted with permission from Ref.[61]. Copyright 2022 Wiley-VCH[61].
Surface modification
Surface modification is an efficient way to prevent the dramatic volume variation[74,114,115], side reaction between cathode and electrolyte[62,116,117] and dissolution of metal ions during charging and discharging[73,118,119]. The common coating layers mainly include oxides, phosphates, fluorides and organic compounds. In comparison to the pristine K0.5MnO2, the Al2O3-modified P3-K0.5MnO2 (K0.5MnO2@Al2O3) achieves a CE exceeding 95%, while the original cathode demonstrates a CE of only 87% after 300 cycles. This enhancement can be ascribed to the effective reduction of side reactions on the interface by the coating layer[73]. Analysis of the electrochemical impedance spectroscopy (EIS) and inductively coupled plasma atomic emission spectroscopy (ICP-AES) results reveals that the K0.5MnO2@Al2O3 electrode exhibits significantly lower RSEI and Rct [Figure 9A], along with decreased levels of dissolved Mn, when contrasted with the K0.5MnO2 electrode, suggesting that coating layer enhances charge transfer efficiency and alleviates structural degradation.
Figure 9. (A) Nyquist plots with the insets of high-frequency semicircles and corresponding equivalent circuits of K0.5MnO2 and
A flexible coating layer is often considered to better mitigate the stress generated by volume variations, thereby minimizing the development of surface cracks. In terms of cycling performance, KNMO modified with amorphous FePO4 (KNMO@a-FP) demonstrates superior stability (66% capacity retention after
Surface modification can not only suppress interfacial side reactions and alleviate mechanical stress but also achieve the reversible phase transition of the cathode. Two types of K0.5Mn0.85Co0.1Fe0.05O2 (KMCF) cathodes coated with KMn4(PO4)3 were obtained through quenching technology, designated as KMCF-P0.1 and KMCF-P0.3, where the numbers 0.1 and 0.3, respectively, represent the concentrations of NH4H2PO4 solutions used[118]. Compared with KMCF and KMCF-P0.3, a better cycling stability was achieved in KMCF-P0.1 whose capacity retained 67.75 mA h g-1 even after cycling 500 cycles at 2 C. In the in-situ XRD spectra, it is found while both KMCF and KMCF-P0.1 experienced comparable phase transition processes, the main peak of KMCF decreases in intensity during the second charge-discharge cycle. Conversely, the high reversibility was maintained in KMCF-P0.1 propelling stable long cycles [Figure 9C]. Surface residual alkali usually exhibits low conductivity, leading to decreased electrochemical performance and compromising air stability of the cathode. Meanwhile, the PVDF can be deactivated by residual alkali, rendering the preparation of the electrode more challenging. Shi et al. developed a method to convert surface residual alkali into a KTaO3 coating layer [Figure 9D], significantly enhancing the cycling stability and air stability of the cathode, while inhibiting the dissolution of transition metal ions[119]. Further, due to the unique ion diffusion path, the kinetic performance was also improved.
Upon exposure to air, layered potassium oxides frequently undergo structural degradation and surface reconstruction as a result of water molecule insertion and K+/H+ ion exchange, significantly affecting electrochemical performance. Constructing a physical barrier on the cathode to block direct contact with air can effectively improve the air stability of the cathode. Cai et al. effectively improved the air stability of
Structure design
The K layered oxides prepared by solid-state methods typically exhibit a flake-like morphology, which usually face some shortcomings including stress concentration, limited contact area, and slow kinetics[121]. Designing cathodes with a distinctive morphology and nano-structure is advantageous in reducing the diffusion barrier of K+, enhancing the electrode/electrolyte contact area, and mitigating volume variation, leading to improved overall performance[122,123].
A layered K0.7Fe0.5Mn0.5O2 nanowire structure was designed via the electrospinning method and sintering[124]. Benefiting from the special interconnected nanowire, it contributes a fast electron/K+ transport channel and a large contact area between electrode and electrolyte, promoting a good rate performance [Figure 10A]. Apart from nanowire structures, some other morphologies such as microspheres[44,125,126], nanosheets[127,128] and microcubes[129] also exhibit a similar effect. The hierarchical structure provides a reliable pathway for K+ ion and electron transportation, as well as buffering the mechanical strain resulting from K+ ion extraction and insertion. K0.65Fe0.5Mn0.5O2 microspheres were designed through the solvent-thermal method[45]. The compact and uniform hierarchical microspheres effectively sustain the stress resulting from volume changes during K+ extraction and insertion, and hasten the diffusion kinetics of K+ by shortening the diffusion distances [Figure 10B]. The cathode delivers an initial specific capacity of about 151 mA h g-1 at an average potential of 2.5 V (vs. K/K+), within the range of 1.5 and 4.2 V at 20 mA g-1. Similarly, compared with irregular K0.5Mn0.8Co0.1Ni0.1O2, the K0.5Mn0.8Co0.1Ni0.1O2 microcuboid exhibits higher surface area with mesopores, which facilitates ion diffusion[129]. Micropores usually show high surface area but may hinder K+ ion diffusion due to size constraints. Macropores enable rapid ion transport but sacrifice capacity owing to the reduced active sites. Mesoporous materials are often seen as the optimal solution for balancing capacity, rate and stability. The Yolk-Shell K0.5Mn0.85Ni0.1Co0.05O2 was synthesized by co-precipitation method with a high specific capacity and excellent cycling performance, which displays the initial capacity of 106 mA h g-1 at 20 mA g-1 corresponding 0.43 K+ insertion in per formula unit and 80.5% capacity retention after 400 cycles at 200 mA g-1[130]. The strain simulation shows that the yolk-shell structure experiences a lower internal strain throughout the sphere with volume changes benefiting excellent battery performance [Figure 10C].
Figure 10. (A) Schematic illustrations of the interconnected K0.7Fe0.5Mn0.5O2 nanowires[124]. Reprinted with permission from Ref.[124]. Copyright 2016 American Chemical Society[124]. (B) Schematic illustration for the synthesis of uniform P2-type K0.65Fe0.5Mn0.5O2 microspheres with hierarchical structure[45]. Reprinted with permission from Ref.[45]. Copyright 2018 Wiley-VCH[45]. (C) The finite element model for strain variation during volume expansion[130]. Reprinted with permission from Ref.[130]. Copyright 2021 Wiley-VCH[130]. Cyclic voltammetry of (D) S-KMO electrode and (E)AlF3@S-KMO electrode[132]. (D and E) Figures Reprinted with permission from Ref.[132]. Copyright 2019 Wiley-VCH[132]. Ex situ XRD patterns of (F) H-K0.7MnO2 and (G) H-K0.7Mn0.7Mg0.3O2[133]. (F and G) Figures reprinted with permission from Ref.[133]. Copyright 2020 Elsevier[133]. (H) long-term cycling stability under 1 C of three compounds[134]. Reprinted with permission from Ref.[134]. Copyright 2024 Wiley-VCH[134].
It is well known that polycrystalline cathode materials are susceptible to cracking due to the uneven distribution of stress on primary particles. Furthermore, the restricted exposure of certain crystal planes hinders the diffusion kinetics in polycrystalline cathodes, resulting in inferior rate performance. Conversely, single-crystal materials benefit from their inherent structural integrity, effectively mitigating structural cracking, while also promoting ion diffusion kinetics through the controlled growth of specific crystal planes. Lv et al. employed the synergistic effect of Fe and Cu elements to synthesize a single-crystal
Combining morphology design and surface coating can prevent side reactions at the interface and enhance structural stability during the charging/discharging process. The strong cationic peak at 2.14 V of
Within potassium-layered oxides, P2-type materials generally demonstrate better structural stability because of their mirror-symmetric structure, while P3-type materials commonly exhibit high specific capacity. Consequently, multiphase composite structures have been designed to harness the synergistic benefits of each phase, achieving superior electrochemical performance[134]. Liu et al. have achieved a series of
Electrolyte optimization
The electrolyte in PIBs plays a pivotal role in dictating the electrochemical performance of layered oxide cathodes. It governs K+ ion transport kinetics, interfacial stability, and the formation of a stable CEI, which directly impacts capacity retention, rate capability, and cycle life[136,137]. Currently, carbonate-based electrolyte such as 0.8 M KPF6 in EC:DEC (1:1) is the most popular electrolyte for PIBs. It is noted that, however, He et al. reported that the highly reactive K metal may react with conventional carbonate solvent resulting in a low CE[138]. Besides, high-voltage electrolytes for layered oxides are still lacking, constraining the performance of electrodes. Therefore, electrolyte optimization, including high-concentration electrolyte (HCEs), locally HCEs, and electrolyte additives, has been widely studied, which are mainly focused on forming a robust CEI.
An inorganic-rich CEI is usually regarded as more stable than an organic CEI because of its high mechanical strength and poor solubility in organic electrolytes. Recently, Zhao et al. reported a high-concentration electrolyte [3 M KFSI in trimethyl phosphate (TMP)][139]. The Raman spectra results identified that there are more contact ion pairs (CIP) and aggregate clusters (AGG) in the TMP-based electrolyte, reducing free solvent molecules and promoting robust inorganic-rich CEI [Figure 11A]. The thin and uniform CEI maintained the long cycling stability for the cathode (85.1% capacity retention at
Figure 11. (A) Raman spectra of three electrolytes[139]. Reprinted with permission from Ref.[139]. Copyright 2024 Wiley-VCH[139]. (B) XPS spectra of C 1s, F 1s, N 1s, and S 2p on K0.67MnO2 surface[142]. Reprinted with permission from Ref.[142]. Copyright 2024 Wiley-VCH[142]. TEM images of SEI on the graphite cycled in 0.8 M KFSI-TFP (C) and 0.8 M KPF6-EC/DEC (D) for 20 and 200 cycles[143].
The weakly coordinated solvent, which facilitates the occupation of anions within the primary solvation structure, enhances the inorganic composition of the CEI. Gu et al., employing a long chain linear ether [dipropylene glycol dimethyl ether (DPGDME)] along with a weakly coordinated solvent [ethylene glycol dibutyl ether (DBE)], prepared 1.5 M KFSI-DPGDME/DBE (designed as MB)[142]. Owing to the special solvation structure, the oxidation potential is promoted to 4.94 V higher than that of a common electrolyte. The results of in-depth XPS further confirm that there are more decompositions of FSI- anions, which possessed high electron conductivity and mechanical strength [Figure 11B]. The transmission electron microscopy (TEM) images illustrate that the MB electrolyte facilitates the formation of a thin and homogeneous CEI, promoting rapid interfacial kinetics. Due to the strong electronegativity of fluorine atoms, fluorine-containing solvents generally have weaker coordination with K+ ions, thus encouraging the participation of more anions in interphase formation[143,144]. The linear sweep voltammetry (LSV) test results for 0.8 M KFSI in TFP reveal a cutoff voltage of up to 4.95 V, significantly higher than that of conventional electrolytes. Moreover, compared to 0.8 M KPF6 in EC:DEC (1:1), the interphase formed on the electrode surface by
Certain beneficial electrolyte additives such as lithium difluoro(oxalate) borate (LiDFOB), KNO3 and AlCl3 also have a positive effect in enhancing the interphase stability, thereby contributing to improved performance[145-148]. The K0.49MnO2 cathode employing 0.8 M KPF6 in EC:DEC (1:1) with 0.7 wt% LiDFOB (BE-LiDFOB) as electrolyte exhibits a superior capacity (more than 120 mA h g-1 at 20 mA g-1 between
SUMMARY AND PERSPECTIVES
PIBs are emerging as promising substitutes for LIBs due to the abundant potassium resources available. However, the K layered oxide cathodes have shown unsatisfactory electrochemical performance [Table 1]. The ideal layered oxide cathode for PIBs should meet the following characteristics: (1) high structural reversibility to minimize phase transition and lattice strain during K+ ions (de)intercalation; (2) High voltage operation through transition metal coordination optimization to achieve high energy density; (3) Air stability to prevent moisture-induced K+/H+ exchange and structural degradation during ambient storage; (4) Excellent electronic/ionic conductivity to enable fast K+ diffusion kinetics; and (5) Robust surface to block electrolyte corrosion and mitigate interfacial side reactions. In this review, the critical issues for K layered oxide cathodes are systematically discussed, including irreversible phase transition, air instability and interfacial instability. Additionally, the corresponding strategies encompassing element doping, surface modification, structural design, and electrolyte optimization have also been provided. More specifically, element doping primarily aims to inhibit phase transitions and the Jahn-Teller effect of Mn3+, although some literature also reports that doped elements can be involved in forming interfacial films to enhance interface characteristics. Surface modification is focused on suppressing side reactions at the interface and the dissolution of transition metals to improve interfacial properties. Structural design emphasizes reducing the structural stress generated by volume expansion and improving ion transport kinetics. Electrolyte optimization seeks to improve the properties of the CEI, reducing the dissolution of transition metals for enhanced structural stability.
A summary of the electrochemical performance of K layered oxide cathodes
| Strategy | Cathode | Voltage window | Specific capacity/mA h g-1 (current density/mA g-1) | Capacity retention/ (cycles, current density/mA g-1) |
| Surface modification | K0.5MnO2@Al2O3[73] | 1.5-4 V | 127.6 (20) | 53% (300, 50) |
| K0.5Ni0.1Mn0.9O2@FePO4[74] | 1.5-3.9 V | 134.7 (20) | 85% (500, 500) | |
| K0.5Mn0.8Co0.2O2@K3PO4/MnPO4[114] | 1.5-3.9 V | 101.3 (100) | 80% (500, 1,000) | |
| K0.5Mn0.9Ti0.1O2@CaTiO3[115] | 1.5-3.9 V | 119.3 (20) | 94.7% (300, 200) | |
| K0.5Mn0.85Co0.1Fe0.05O2@KMn4(PO4)3[118] | 1.5-4 V | 100.5 (20) | 72.97% (500, 200) | |
| K1.39Mn3O6@AlF3[132] | 1.5-4 V | 110 (10) | 94.9% (100, 50) | |
| K0.5MnO2@KTaO3[119] | 1.5-3.9 V | 82.07 (400) | 98.1% (100, 100) | |
| Structure design | P3-K0.5MnO2 microsphere[44] | 1.5-3.9 V | 104 (10) | 89.1% (400, 200) |
| P3/P2-K0.37Na0.3Ni0.17Co0.17Mn0.66O2[135] | 2-4.2 V | 86.12 (20) | 91.5% (100, 20) | |
| P2-K0.65Fe0.5Mn0.5O2 microsphere[45] | 1.5-4.2 V | 151 (20) | 78% (350, 100) | |
| P2-K0.6CoO2 microsphere[121] | 1.7-4 V | 82 (10) | 87% (300, 40) | |
| Hierarchical K0.7Mn0.7Mg0.3O2[133] | 1.5-4 V | 144.5 (20) | 82.5% (400, 100) | |
| P3-K0.5Mn0.8Ni0.1Co0.1O2 microcuboid[129] | 1.5-4 V | 94.5 (20) | 61% (300, 100) | |
| P3-K0.45Mn0.5Co0.5O2 peanut[127] | 1.2-3.9 V | 123.2 (20) | 73.8% (300, 300) | |
| P2-K0.6CoO2 microcube[122] | 1.7-4 V | 87.2 (20) | 86.9% (1,000, 40) | |
| P'3-K0.35Mn0.8Fe0.1Cu0.1O2 (single crystalline)[131] | 1.5-3.9 V | 96.8 (50) | 91.1% (500, 500) | |
| P2/P3-K0.7Mn0.67Ni0.33O2[134] | 1.5-4 V | 116.3 (10) | 83.1% (600, 100) | |
| O3/P2/P3-Na0.9Ni0.3Mn0.55Cu0.1Ti0.05O2[161] | 1.5-4 V | 108 (15) | 83% (600, 300) | |
| Element doping | P3-K0.5Mn0.8Fe0.1Ni0.1O2[79] | 1.5-3.9 V | 120 (50) | 74% (300, 50) |
| P3-K0.5Ni0.1Mn0.9O2[24] | 1.5-3.9 V | 121 (10) | 82% (100, 100) | |
| P3-K0.54Co0.5Mn0.5O2[82] | 1.5-3.9 V | 120.4 (20) | 62% (500, 20) | |
| K0.28MnO2·0.15H2O[100] | 1.4-3.6 V | 152 (15) | 81% (100, 15) | |
| P2-K0.44Ni0.22Mn0.78O2[41] | 1.5-4 V | 125.5 (10) | 67% (500, 200) | |
| P3-K0.45Ni0.1Co0.1Al0.05Mn0.75O2[166] | 1.5-4 V | 84.5 (20) | 77.4% (100, 20) | |
| P3-K0.67Mn0.83Ni0.17O2[42] | 1.5-3.8 V | 122 (20) | 75% (200, 500) | |
| K5/9Mn7/9Ti2/9O2[91] | 1.5-4.2 V | 135 (20) | 70% (100, 100) | |
| P'3-K0.3Mn0.9Cu0.1O2[111] | 1.5-3.9 V | 124 (10) | 82% (50, 100) | |
| P3-K0.5Mn0.6Co0.2Fe0.1Mg0.1O2[92] | 1.5-3.9 V | 104.6 (50) | 91% (150, 100) | |
| P3-K0.4Mn0.8Fe0.1Ti0.1O2[86] | 1.8-4 V | 117 (20) | 74% (300, 200) | |
| P3-K0.45Co1/12Mg1/12Mn5/6O2[157] | 1.5-3.9 V | 98 (120) | 54.8% (500, 1,000) | |
| P3-K0.54Mn0.78Mg0.22O2[90] | 1.5-4 V | 132.4 (20) | 84% (100, 200) | |
| P3-K0.45Rb0.05Mn0.85Mg0.15O2[104] | 1.5-3.9 V | 108.0 (20) | 98.2% (200, 200) | |
| K0.4Fe0.1Mn0.8Ti0.1O2·0.16H2O[61] | 1.8-4 V | 116 (20) | 90% (1,000, 2,000) | |
| P3-K0.45Mn0.9Al0.1O2[112] | 1.5-3.9 V | 152 (20) | 67% (1,000, 500) | |
| P'3-K0.6MnO1.97F0.03[96] | 1.5-3.9 V | 111 (10) | 77.3% (100, 100) | |
| K0.4Mn0.9Li0.1O2·0.33H2O[102] | 1.5-4 V | 101.87 (20) | 84.04% (100, 100) | |
| P2-K0.02Na0.55Mn0.7Ni0.25Zn0.05O2[167] | 1.5-4 V | 57 (100) | 97% (1,000, 100) | |
| P3-K0.6Ni0.05Fe0.05Mg0.05Ti0.05Mn0.0725O2[108] | 1.5-3.9 V | 39.3 (2,000) | 87.5% (600, 1,000) | |
| P3-K0.7Fe0.05Co0.1Mn0.75Ni0.05V0.05O2[106] | 1.5-3.9 V | 77.39 (1,000) | 70.3% (500, 1,000) | |
| P3-K0.45Mn0.6Ni0.075Fe0.075Co0.075Ti0.1Cu0.05Mg0.025O2[107] | 1.5-4.2 V | 106.8 (10) | 60.4% (200, 100) | |
| P3-K0.5Mn0.8Co0.2B0.1O2[98] | 1.4-4 V | 121.4 (10) | 87.1% (100, 200) | |
| P'3-K0.5Mn0.8Cu0.1Mg0.1O2[89] | 1.5-4 V | 100.1 (10) | 75.41% (100, 50) | |
| P3-K0.39Mn0.77Ni0.23O1.9F0.1[95] | 1.5-4 V | 93.5 (20) | 77% (350, 100) | |
| P3-K0.5Mn0.8Co0.1Zn0.1O2[168] | 1.5-3.9 V | 105 (100) | 89.8% (100, 100) | |
| P3-K0.57Cu0.1Fe0.1Mn0.8O2[169] | 1.5-4.2 V | 135 (20) | 68% (500, 200) | |
| P3-K0.5Mn0.87Co0.05Fe0.05Li0.03O2[94] | 1.5-4 V | 107.1 (20) | 88.5% (300, 200) | |
| P3-K0.67Li0.07Mn0.93O2[93] | 1.5-4 V | 84.3 (10) | 81.3% (100, 50) | |
| P2-K0.6Mn0.8Ni0.1Ti0.1O2[170] | 1.5-4.2 V | 118 (10) | 88% (100, 200) | |
| P3-K0.5Mg0.15Mn0.8Mg0.05O2[103] | 1.4-4 V | 102.0 (10) | 48.2% (400, 100) | |
| P2-K0.56Na0.11Li0.12Ni0.22Mn0.66O2[46] | 1.5-4.6 V | 90.2 (20) | 89.6% (150, 20) |
While significant progress has been made in K layered oxide research, the field is still in its early stages, with incomplete understanding of the underlying mechanisms. The kinetics evolution at the interface and the relationship between material structure and performance still require further investigation. To address this, advanced in-situ/ex-situ characterization techniques and machine learning-guided simulations should be prioritized to map the kinetics evolution at interfaces and establish structure-property correlations[149,150]. Besides, despite K metal sharing a comparable standard hydrogen electrode potential with Li metal, its high atomic weight results in a lower specific capacity. Importantly, many K layered oxides exhibit potassium deficiency, further compromising their electrochemical performance. Emerging strategies such as pre-potassiation or using potassium-rich self-sacrificial additives (e.g., K2C4O4) could mitigate the inherent potassium deficiency in layered oxides[14,151]. The coin cell tests for PIBs are typically conducted over a wide voltage range (1.5-4 V) to maximize capacity. However, the lower discharge voltage necessitates the occurrence of Mn3+/Mn4+ redox, potentially weakening structural stability due to the Jahn-Teller effect of high-spin Mn3+. Elemental doping
DECLARATIONS
Acknowledgments
Prof. Wang, P. F. thanks the support from Young Elite Scientists Sponsorship Program by the Chinese Association for Science and Technology, the “High-Level Talent Introduction Plan” of Shaanxi Province, Siyuan Scholar and Xiaomi Youth Scholar of Xi'an Jiaotong University.
Authors’ contributions
Conducted the literature review and wrote the manuscript: Tang, W.; Tang, Y.
Revised: Liu, M.; Cheng, Y.; Wang, P. F.; Tang, W.
Reviewed and edited the manuscript: Wang, P. F.
All authors participated in preparing the manuscript.
Availability of data and materials
Not applicable.
Financial support and sponsorship
This work was supported by the National Natural Science Foundation of China (Grant Nos. 52472249 and 22409161), the Young Talent Support Plan of Xi'an Jiaotong University (Grant No. DQ6J011), and the State Key Laboratory of Electrical Insulation and Power Equipment (Grant No. EIPE23313).
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Choi, J. W.; Aurbach, D. Promise and reality of post-lithium-ion batteries with high energy densities. Nat. Rev. Mater. 2016, 1, 201613.
2. Kim, H.; Seo, D. H.; Bianchini, M.; et al. A new strategy for high-voltage cathodes for K-ion batteries: stoichiometric KVPO4F. Adv. Energy. Mater. 2018, 8, 1801591.
3. Gurung, A.; Qiao, Q. Solar charging batteries: advances, challenges, and opportunities. Joule 2018, 2, 1217-30.
4. Lee, W.; Kim, J.; Yun, S.; Choi, W.; Kim, H.; Yoon, W. Multiscale factors in designing alkali-ion (Li, Na, and K) transition metal inorganic compounds for next-generation rechargeable batteries. Energy. Environ. Sci. 2020, 13, 4406-49.
5. Vaalma, C.; Buchholz, D.; Weil, M.; Passerini, S. A cost and resource analysis of sodium-ion batteries. Nat. Rev. Mater. 2018, 3, 18013.
6. Liu, Y.; Sun, Z.; Tan, K.; et al. Recent progress in flexible non-lithium based rechargeable batteries. J. Mater. Chem. A. 2019, 7, 4353-82.
7. Sultana, I.; Ramireddy, T.; Rahman, M. M.; Chen, Y.; Glushenkov, A. M. Tin-based composite anodes for potassium-ion batteries. Chem. Commun. 2016, 52, 9279-82.
8. Xu, Y.; Sun, J.; He, Y.; et al. Construction of CoS2 nanoparticles embedded in well-structured carbon nanocubes for high-performance potassium-ion half/full batteries. Sci. China. Chem. 2021, 64, 1401-9.
9. Komaba, S.; Hasegawa, T.; Dahbi, M.; Kubota, K. Potassium intercalation into graphite to realize high-voltage/high-power potassium-ion batteries and potassium-ion capacitors. Electrochem. Commun. 2015, 60, 172-5.
10. Okoshi, M.; Yamada, Y.; Komaba, S.; Yamada, A.; Nakai, H. Theoretical analysis of interactions between potassium ions and organic electrolyte solvents: a comparison with lithium, sodium, and magnesium ions. J. Electrochem. Soc. 2017, 164, A54-60.
12. Wang, B.; Ang, E. H.; Yang, Y.; et al. Post-lithium-ion battery era: recent advances in rechargeable potassium-ion batteries. Chemistry 2021, 27, 512-36.
13. Kim, H.; Ji, H.; Wang, J.; Ceder, G. Next-generation cathode materials for non-aqueous potassium-ion batteries. Trends. Chem. 2019, 1, 682-92.
14. Saju, S. K.; Chattopadhyay, S.; Xu, J.; Alhashim, S.; Pramanik, A.; Ajayan, P. M. Hard carbon anode for lithium-, sodium-, and potassium-ion batteries: advancement and future perspective. Cell. Rep. Phys. Sci. 2024, 5, 101851.
15. Pramanik, A.; Sengupta, S.; Saju, S. K.; Chattopadhyay, S.; Kundu, M.; Ajayan, P. M. Ternary metal sulfides as electrode materials for Na/K-ion batteries and electrochemical supercapacitor: advances/challenges and prospects. Adv. Energy. Mater. 2024, 14, 2401657.
16. Wang, X.; Luo, Z.; Huang, J.; et al. S/N-co-doped graphite nanosheets exfoliated via three-roll milling for high-performance sodium/potassium ion batteries. J. Mater. Sci. Technol. 2023, 147, 47-55.
17. Vaalma, C.; Giffin, G. A.; Buchholz, D.; Passerini, S. Non-aqueous K-ion battery based on layered K0.3MnO2 and hard carbon/carbon black. J. Electrochem. Soc. 2016, 163, A1295.
18. Park, W. B.; Han, S. C.; Park, C.; et al. KVP2O7 as a robust high-energy cathode for potassium-ion batteries: pinpointed by a full screening of the inorganic registry under specific search conditions. Adv. Energy. Mater. 2018, 8, 1703099.
19. Zhang, X.; Wei, Z.; Dinh, K. N.; et al. Layered oxide cathode for potassium-ion battery: recent progress and prospective. Small 2020, 16, e2002700.
20. Zhu, Y. H.; Zhang, Q.; Yang, X.; et al. Reconstructed orthorhombic V2O5 polyhedra for fast ion diffusion in K-ion batteries. Chem 2019, 5, 168-79.
21. Linnell, S. F.; Kim, E. J.; Choi, Y. S.; et al. Enhanced oxygen redox reversibility and capacity retention of titanium-substituted
22. Hosaka, T.; Kubota, K.; Hameed, A. S.; Komaba, S. Research development on K-ion batteries. Chem. Rev. 2020, 120, 6358-466.
23. Kaufman, J. L.; Van der Ven, A. Cation diffusion facilitated by antiphase boundaries in layered intercalation compounds. Chem. Mater. 2022, 4, 1889-96.
24. Cho, M. K.; Jo, J. H.; Choi, J. U.; et al. Cycling stability of layered potassium manganese oxide in nonaqueous potassium cells. ACS. Appl. Mater. Interfaces. 2019, 11, 27770-9.
25. Wu, Z.; Zou, J.; Chen, S.; Niu, X.; Liu, J.; Wang, L. Potassium-ion battery cathodes: past, present, and prospects. J. Power. Sources. 2021, 484, 229307.
26. Cong, J.; Luo, S. H.; Li, K.; et al. Research progress of manganese-based layered oxides as cathode materials for potassium-ion batteries. J. Electroanal. Chem. 2022, 927, 116971.
27. Xu, Y. S.; Guo, S. J.; Tao, X. S.; et al. High-performance cathode materials for potassium-ion batteries: structural design and electrochemical properties. Adv. Mater. 2021, 33, e2100409.
28. Delmas, C.; Fouassier, C.; Hagenmuller, P. Evolution cristallochimique et propriétés physiques de quelques oxydes lamellaires. Mater. Sci. Eng. 1977, 31, 297-301.
29. Delmas, C.; Fouassier, C.; Hagenmuller, P. Structural classification and properties of the layered oxides. Phys. B+C. 1980, 99, 81-5.
30. Ren, M.; Fang, H.; Wang, C.; et al. Advances on manganese-oxide-based cathodes for Na-ion batteries. Energy. Fuels. 2020, 34, 13412-26.
31. Wang, P. F.; You, Y.; Yin, Y. X.; Guo, Y. Layered oxide cathodes for sodium-ion batteries: phase transition, air stability, and performance. Adv. Energy. Mater. 2018, 8, 1701912.
32. Huang, Z. X.; Gu, Z. Y.; Heng, Y. L.; Ang, E. H.; Geng, H. B.; Wu, X. L. Advanced layered oxide cathodes for sodium/potassium-ion batteries: development, challenges and prospects. Chem. Eng. J. 2023, 452, 139438.
33. Jha, P. K.; Pralong, V.; Fichtner, M.; Barpanda, P. P3 type layered oxide frameworks: an appealing family of insertion materials for K-ion batteries. Curr. Opin. Electrochem. 2023, 38, 101216.
34. Jha, P. K.; Totade, S. N.; Barpanda, P.; et al. Evaluation of P3-type layered oxides as K-ion battery cathodes. Inorg. Chem. 2023, 62, 14971-9.
35. Liao, J.; Han, Y.; Zhang, Z.; Xu, J.; Li, J.; Zhou, X. Recent progress and prospects of layered cathode materials for potassium-ion batteries. Energy. Environ. Mater. 2021, 4, 178-200.
36. Liu, Z.; Su, H.; Yang, Y.; Wu, T.; Sun, S.; Yu, H. Advances and perspectives on transitional metal layered oxides for potassium-ion battery. Energy. Storage. Mater. 2021, 34, 211-28.
37. Liu, C. L.; Luo, S. H.; Huang, H. B.; Zhai, Y. C.; Wang, Z. W. Layered potassium-deficient P2- and P3-type cathode materials KxMnO2 for K-ion batteries. Chem. Eng. J. 2019, 356, 53-9.
38. Kim, H.; Kim, J. C.; Bo, S. H.; Shi, T.; Kwon, D. H.; Ceder, G. K-ion batteries based on a P2-type K0.6CoO2 cathode. Adv. Energy. Mater. 2017, 7, 1700098.
39. Hwang, J. Y.; Kim, J.; Yu, T. Y.; Myung, S. T.; Sun, Y. K. Development of P3-K0.69CrO2 as an ultra-high-performance cathode material for K-ion batteries. Energy. Environ. Sci. 2018, 11, 2821-7.
40. Kim, H.; Seo, D. H.; Urban, A.; et al. Stoichiometric layered potassium transition metal oxide for rechargeable potassium batteries. Chem. Mater. 2018, 30, 6532-9.
41. Zhang, X.; Yang, Y.; Qu, X.; et al. Layered P2-Type K0.44Ni0.22Mn0.78O2 as a high-performance cathode for potassium-ion batteries. Adv. Funct. Mater. 2019, 29, 1905679.
42. Bai, P.; Jiang, K.; Zhang, X.; Xu, J.; Guo, S.; Zhou, H. Ni-doped layered manganese oxide as a stable cathode for potassium-ion batteries. ACS. Appl. Mater. Interfaces. 2020, 12, 10490-5.
43. Xiao, Z.; Meng, J.; Xia, F.; et al. K+ modulated K+/vacancy disordered layered oxide for high-rate and high-capacity potassium-ion batteries. Energy. Environ. Sci. 2020, 13, 3129-37.
44. Peng, B.; Li, Y.; Gao, J.; Zhang, F.; Li, J.; Zhang, G. High energy K-ion batteries based on P3-Type K0.5MnO2 hollow submicrosphere cathode. J. Power. Sources. 2019, 437, 226913.
45. Deng, T.; Fan, X.; Chen, J.; et al. Layered P2-type K0.65Fe0.5Mn0.5O2 microspheres as superior cathode for high-energy potassium-ion batteries. Adv. Funct. Mater. 2018, 28, 1800219.
46. Tang, Y.; Dong, H.; Liu, M.; et al. Realizing a single-phase reaction and K+/vacancy disordering in P2-
47. Nathan, M. G. T.; Park, W. B.; Naveen, N.; et al. A comparison of as-synthesized P2-K0.70[Cr0.85Sb0.15]O2 and Ion-Exchanged P2-
48. Park, H.; Lee, Y.; Ko, W.; et al. Review on cathode materials for sodium- and potassium-ion batteries: structural design with electrochemical properties. Batteries. Supercaps. 2023, 6, e202200486.
49. Li, W.; Bi, Z.; Zhang, W.; et al. Advanced cathodes for potassium-ion batteries with layered transition metal oxides: a review. J. Mater. Chem. A. 2021, 9, 8221-47.
50. Yabuuchi, N.; Kubota, K.; Dahbi, M.; Komaba, S. Research development on sodium-ion batteries. Chem. Rev. 2014, 114, 11636-82.
51. Sun, K.; Luo, S. H.; Hao, G.; et al. Review on layered manganese-based metal oxides cathode materials for potassium-ion batteries: from preparation to modification. Chem. Rec. 2024, 24, e202300327.
52. Kim, H.; Seo, D. H.; Kim, J. C.; et al. Investigation of potassium storage in layered P3-type K0.5MnO2 cathode. Adv. Mater. 2017, 29, 1702480.
53. Liu, Y. F.; Han, K.; Peng, D. N.; et al. Layered oxide cathodes for sodium-ion batteries: from air stability, interface chemistry to phase transition. InfoMat 2023, 5, e12422.
54. Su, Y.; Li, L.; Chen, G.; et al. Strategies of removing residual lithium compounds on the surface of Ni-rich cathode materials. Chin. J. Chem. 2021, 39, 189-98.
55. Seong, W. M.; Kim, Y.; Manthiram, A. Impact of residual lithium on the adoption of high-nickel layered oxide cathodes for lithium-ion batteries. Chem. Mater. 2020, 32, 9479-89.
56. Yao, H. R.; Zheng, L.; Xin, S.; Guo, Y. G. Air-stability of sodium-based layered-oxide cathode materials. Sci. China. Chem. 2022, 65, 1076-87.
57. Lu, Z.; Dahn, J. R. Intercalation of water in P2, T2 and O2 structure Az[CoxNi1/3-xMn2/3]O2. Chem. Mater. 2001, 13, 1252-7.
58. Han, M. H.; Gonzalo, E.; Sharma, N.; et al. High-performance P2-phase Na2/3Mn0.8Fe0.1Ti0.1O2 cathode material for ambient-temperature sodium-ion batteries. Chem. Mater. 2016, 28, 106-16.
59. Duffort, V.; Talaie, E.; Black, R.; Nazar, L. F. Uptake of CO2 in layered P2-Na0.67Mn0.5Fe0.5O2: insertion of carbonate anions. Chem. Mater. 2015, 27, 2515-24.
60. Yang, Y.; Wang, Z.; Du, C.; et al. Decoupling the air sensitivity of Na-layered oxides. Science 2024, 385, 744-52.
61. Zhang, X.; Yang, X.; Sun, G.; et al. Hydration enables air-stable and high-performance layered cathode materials for both organic and aqueous potassium-ion batteries. Adv. Funct. Mater. 2022, 32, 2204318.
62. Liu, X.; Guo, Y.; Zhang, Q.; et al. K-rich spinel interface of air-stable layered oxide cathodes for potassium-ion batteries. Adv. Mater. 2024, 36, e2407980.
63. Wang, H.; Zhai, D.; Kang, F. Solid electrolyte interphase (SEI) in potassium ion batteries. Energy. Environ. Sci. 2020, 13, 4583-608.
64. Mao, J.; Wang, C.; Lyu, Y.; et al. Organic electrolyte design for practical potassium-ion batteries. J. Mater. Chem. A. 2022, 10, 19090-106.
65. Liu, Y.; Gao, C.; Dai, L.; et al. The features and progress of electrolyte for potassium ion batteries. Small 2020, 16, e2004096.
66. Zhang, J.; Gai, J.; Song, K.; Chen, W. Advances in electrode/electrolyte interphase for sodium-ion batteries from half cells to full cells. Cell. Rep. Phys. Sci. 2022, 3, 100868.
67. Shi, C.; Wang, L.; Chen, X.; et al. Challenges of layer-structured cathodes for sodium-ion batteries. Nanoscale. Horiz. 2022, 7, 338-51.
68. Che, H.; Yang, X.; Wang, H.; et al. Long cycle life of sodium-ion pouch cell achieved by using multiple electrolyte additives. J. Power. Sources. 2018, 407, 173-9.
69. Wang, C.; Wang, K.; Ren, M.; et al. Interfacial chemistry enables highly reversible Na extraction/intercalation in layered-oxide cathode materials. Chin. J. Chem. 2023, 41, 1791-6.
71. Mu, L.; Feng, X.; Kou, R.; et al. Deciphering the cathode-electrolyte interfacial chemistry in sodium layered cathode materials. Adv. Energy. Mater. 2018, 8, 1801975.
72. Yaghoobnejad Asl H, Manthiram A. Proton-induced disproportionation of Jahn-Teller-Active transition-metal ions in oxides due to electronically driven lattice instability. J. Am. Chem. Soc. 2020, 142, 21122-30.
73. Li, F.; Gu, X.; Wu, S.; et al. Interface engineering enabled high-performance layered P3-type K0.5MnO2 cathode for low-cost potassium-ion batteries. Electrochim. Acta. 2023, 439, 141571.
74. Huang, Y.; Zhang, X.; Chen, N.; Tian, R.; Zeng, Y.; Du, F. A conformal protective skin producing stable cathode-electrolyte interface for long-life potassium-ion batteries. Small 2023, 19, e2302841.
75. Xue, L.; Li, Y.; Gao, H.; et al. Low-cost high-energy potassium cathode. J. Am. Chem. Soc. 2017, 139, 2164-7.
76. Bao, J.; Deng, W.; Liu, J.; Sun, C. Ultrafast-kinetics, ultralong-cycle-life, bifunctional inorganic open-framework for potassium-ion batteries. Energy. Storage. Mater. 2021, 42, 806-14.
77. Liu, T.; Hou, S.; Li, Y.; et al. Insight of K-deficient layered KxMnO2 cathode for potassium-ions batteries. J. Energy. Chem. 2022, 64, 335-43.
78. Puneeth, N. P. N.; Kaushik, S. D.; Kalai Selvan, R. Synthesis and electrochemical properties of crystalline K0.7MnO2 particles for K-ion batteries. Mater. Lett. 2022, 316, 131997.
79. Choi, J. U.; Kim, J.; Jo, J. H.; et al. Facile migration of potassium ions in a ternary P3-type K0.5[Mn0.8Fe0.1Ni0.1]O2 cathode in rechargeable potassium batteries. Energy. Storage. Mater. 2020, 25, 714-23.
80. Baskar, S.; Sada, K.; Barpanda, P. Layered P2-NaxCoO2 and NaxFeO2 as cathode materials for potassium-ion batteries. ECS. Trans. 2017, 80, 357-64.
81. Han, S. C.; Park, W. B.; Sohn, K. S.; Pyo, M. KFeO2 with corner-shared FeO4 frameworks as a new type of cathode material in potassium-ion batteries. J. Solid. State. Electrochem. 2019, 23, 3135-43.
82. Choi, J. U.; Kim, J.; Hwang, J. Y.; Jo, J. H.; Sun, Y. K.; Myung, S. T. K0.54[Co0.5Mn0.5]O2: new cathode with high power capability for potassium-ion batteries. Nano. Energy. 2019, 61, 284-94.
83. Hironaka, Y.; Kubota, K.; Komaba, S. P2- and P3-KxCoO2 as an electrochemical potassium intercalation host. Chem. Commun. 2017, 53, 3693-6.
84. Jha, P. K.; Barpanda, P. Role of Co content on the electrode properties of P3-type K0.5Mn1-xCoxO2 potassium insertion materials. Inorg. Chem. 2024, 63, 7137-45.
85. Puneeth, N. P. N.; Kaushik, S. D.; Kalai, Selvan. R. Improved K-ion diffusion kinetics of cobalt-substituted P3-type K0.67MnO2 electrodes for K-ion batteries. ACS. Appl. Energy. Mater. 2024, 7, 2600-13.
86. Zhang, X.; Yu, D.; Wei, Z.; et al. Layered P3-type K0.4Fe0.1Mn0.8Ti0.1O2 as a low-cost and zero-strain electrode material for both potassium and sodium storage. ACS. Appl. Mater. Interfaces. 2021, 13, 18897-904.
87. Liu, C. L.; Luo, S. H.; Huang, H. B.; Zhai, Y. C.; Wang, Z. W. Low-cost layered K0.45Mn0.9Mg0.1O2 as a high-performance cathode material for K-ion batteries. ChemElectroChem 2019, 6, 2308-15.
88. Ko, W.; Lee, S.; Park, H.; et al. Structural and electrochemical stabilization enabling high-energy P3-type Cr-based layered oxide cathode for K-ion batteries. Carbon. Energy. 2024, 6, e454.
89. Chen, H.; Gao, X. W.; Li, Q.; et al. Layer-structured K0.5Mn0.8Cu0.1Mg0.1O2 for high-performance potassium-ion batteries by alleviating the phase transformation. J. Mater. Chem. A. 2024, 12, 6261-8.
90. Huang, R.; Xue, Q.; Lin, J.; et al. Layered K0.54Mn0.78Mg0.22O2 as a high-performance cathode material for potassium-ion batteries. Nano. Res. 2022, 15, 3143-9.
91. Xu, Y. S.; Zhang, Q. H.; Wang, D.; et al. Enabling reversible phase transition on K5/9Mn7/9Ti2/9O2 for high-performance potassium-ion batteries cathodes. Energy. Storage. Mater. 2020, 31, 20-6.
92. Xiao, Z.; Xia, F.; Xu, L.; et al. Suppressing the Jahn-Teller effect in Mn-based layered oxide cathode toward long-life potassium-ion batteries. Adv. Funct. Mater. 2022, 32, 2108244.
93. Yin, X.; Gu, M.; Yang, Q.; Lei, K. Suppression of Jahn-Teller distortion in a layered Mn-based oxide cathode with Li substitution toward achieving stable K-storage. New. J. Chem. 2024, 48, 9352-7.
94. Gao, X. W.; Wang, S. S.; Li, Q.; Yang, R.; Liu, Z.; Luo, W. B. A synergistic pinning effect in a layer-structured oxide cathode for enhancing stability towards potassium-ion batteries. J. Mater. Chem. A. 2024, 12, 15676-84.
95. Zhang, Z.; Qiao, Y.; Zhao, J.; et al. Fluorine-doped K0.39Mn0.77Ni0.23O1.9F0.1 microspheres with highly reversible oxygen redox reaction for potassium-ion battery cathode. Chin. Chem. Lett. 2025, 36, 109907.
96. Kim, Y.; Oh, G.; Lee, J.; et al. Stabilization of layered-type potassium manganese oxide cathode with fluorine treatment for high-performance K-ion batteries. J. Power. Sources. 2023, 588, 233729.
97. Xu, Y. S.; Qi, M. Y.; Zhang, Q. H.; et al. Anion doping for layered oxides with a solid-solution reaction for potassium-ion battery cathodes. ACS. Appl. Mater. Interfaces. 2022, 14, 13379-87.
98. Xu, X.; Li, X. L.; Rahman, M. M.; et al. Promoting reversibility of layered potassium cathode through interstitial doping. Chem. Eng. J. 2023, 477, 147021.
99. Wu, L.; Fu, H.; Lyu, W.; et al. Rational regulation of high-voltage stability in potassium layered oxide cathodes. ACS. Nano. 2024, 18, 13415-27.
100. Jo, J. H.; Hwang, J. Y.; Choi, J.; Sun, Y. K.; Myung, S. T. Layered K0.28MnO2·0.15H2O as a cathode material for potassium-ion intercalation. ACS. Appl. Mater. Interfaces. 2019, 11, 43312-9.
101. Lin, B.; Zhu, X.; Fang, L.; et al. Birnessite nanosheet arrays with high K content as a high-capacity and ultrastable cathode for K-ion batteries. Adv. Mater. 2019, 31, e1900060.
102. Zhao, Z.; Sun, Y.; Pan, Y.; et al. A new Mn-based layered cathode with enlarged interlayer spacing for potassium ion batteries. J. Colloid. Interface. Sci. 2023, 652, 231-9.
103. Luo, R. J.; Li, X. L.; Ding, J. Y.; et al. Suppressing Jahn-Teller distortion and phase transition of K0.5MnO2 by K-site Mg substitution for potassium-ion batteries. Energy. Storage. Mater. 2022, 47, 408-14.
104. Caixiang, Z.; Hao, J.; Zhou, J.; Yu, X.; Lu, B. Interlayer-engineering and surface-substituting manganese-based self-evolution for high-performance potassium cathode. Adv. Energy. Mater. 2023, 13, 2203126.
105. Zhao, C.; Ding, F.; Lu, Y.; Chen, L.; Hu, Y. S. High-entropy layered oxide cathodes for sodium-ion batteries. Angew. Chem. Int. Ed. 2020, 59, 264-9.
106. Ding, X.; Wang, Y.; Wang, X.; et al. Multi-element doping induced transition metal disordered layered oxide for rapid and stable potassium storage. Chem. Eng. J. 2023, 466, 143331.
107. Chu, S.; Shao, C.; Tian, J.; et al. High entropy-induced kinetics improvement and phase transition suppression in K-ion battery layered cathodes. ACS. Nano. 2024, 18, 337-46.
108. Cai, Y.; Liu, W.; Chang, F.; et al. Entropy-stabilized layered K0.6Ni0.05Fe0.05Mg0.05Ti0.05Mn0.725O2 as a high-rate and stable cathode for potassium-ion batteries. ACS. Appl. Mater. Interfaces. 2023, 15, 48277-86.
109. Zeng, G.; Liu, B.; Ali, U.; et al. The local disorder induced by high-entropy doping results in highly stable cathode materials for aqueous potassium-ion batteries. Appl. Catal. B. Environ. Energy. 2024, 351, 123996.
110. Liu, B.; Zhang, Q.; Zhang, L.; Yong, X.; Li, L.; Wang, C. Manganese charge redistribution induced by high-entropy charge compensation mechanism for aqueous potassium-ion batteries. Energy. Storage. Mater. 2024, 66, 103221.
111. Park, S.; Park, S.; Park, Y.; Alfaruqi, M. H.; Hwang, J.; Kim, J. A new material discovery platform of stable layered oxide cathodes for K-ion batteries. Energy. Environ. Sci. 2021, 14, 5864-74.
112. Huang, Y.; Zhang, X.; Lin, H.; et al. S Synergistically enhanced structural, thermal and interfacial stability of K0.45MnO2 via tailoring the local structure for high-energy and high-power potassium-ion batteries. Chem. Eng. J. 2023, 453, 139571.
113. Mu, L.; Xu, S.; Li, Y.; et al. Prototype sodium-ion batteries using an air-stable and Co/Ni-Free O3-layered metal oxide cathode. Adv. Mater. 2015, 27, 6928-33.
114. Wang, H.; Peng, H.; Xiao, Z.; et al. Double-layer phosphates coated Mn-based oxide cathodes for highly stable potassium-ion batteries. Energy. Storage. Mater. 2023, 58, 101-9.
115. Chen, J.; Rao, A. M.; Gao, C.; et al. Phase-transition-free rivets for layered oxide potassium cathodes. Nano. Res. 2024, 17, 9671-8.
116. Deng, Q.; Tian, C.; Luo, Z. Atomic layer deposition of Al2O3 on organic potassium terephthalate with enhanced K-storage behavior for K-ion batteries. Ionics 2020, 26, 1805-12.
117. Fu, Q.; Peng, C.; Zhou, W.; et al. Regulating cathode surface hydroxyl chemistry enables superior potassium storage. Proc. Natl. Acad. Sci. USA. 2023, 120, e2301622120.
118. Li, S.; Zhao, L. K.; Bian, Y. H.; et al. Protective layer constructed by liquid phase quenching for long lifespan potassium ion batteries. Chem. Eng. J. 2024, 496, 154382.
119. Shi, H.; Gao, X. W.; Wang, X.; et al. Surface residual alkali reverse utilization: stabilizing the lay-structured oxide cathode for high stability potassium ion batteries. Chem. Eng. J. 2024, 484, 149574.
120. Cai, Y.; Zeng, X.; Pang, D.; et al. Layered transition metal oxides prepared by plasma-enhanced sintering technique as environmentally stable cathode for potassium-ion batteries. Materialia 2023, 27, 101674.
121. Deng, T.; Fan, X.; Luo, C.; et al. Self-templated formation of P2-type K0.6CoO2 microspheres for high reversible potassium-ion batteries. Nano. Lett. 2018, 18, 1522-9.
122. Zhang, Z.; Hu, Q.; Liao, J.; et al. Uniform P2-K0.6CoO2 microcubes as a high-energy cathode material for potassium-ion batteries. Nano. Lett. 2023, 23, 694-700.
123. Chen, L.; Zhang, Y.; Hao, C.; et al. Interlayer engineering of KxMnO2 enables superior alkali metal ion storage for advanced hybrid capacitors. ChemElectroChem 2022, 9, e202200059.
124. Wang, X.; Xu, X.; Niu, C.; et al. Earth abundant Fe/Mn-based layered oxide interconnected nanowires for advanced K-ion full batteries. Nano. Lett. 2017, 17, 544-50.
125. Chong, S.; Wu, Y.; Chen, Y.; et al. Mn-based layered oxide microspheres assembled by ultrathin nanosheets as cathode material for potassium-ion batteries. Electrochim. Acta. 2019, 293, 299-306.
126. Xu, S.; Bao, C.; Yu, M.; Liu, S.; Chen, L.; Zhang, D. Layered P3 type K0.48Ni0.2Co0.2Mn0.6O2 with microspherical and microcubic mixed morphology as a cathode material for potassium-ion batteries. Mater. Lett. 2020, 270, 127733.
127. Zhang, Z.; Sun, J.; Duan, L.; et al. Self-templated construction of peanut-like P3-type K0.45Mn0.5Co0.5O2 for highly reversible potassium storage. J. Mater. Chem. A. 2022, 10, 554-60.
128. Duan, L.; Xu, J.; Xu, Y.; et al. Cocoon-shaped P3-type K0.5Mn0.7Ni0.3O2 as an advanced cathode material for potassium-ion batteries. J. Energy. Chem. 2023, 76, 332-8.
129. Duan, L.; Xu, Y.; Zhang, Z.; et al. A high-performance cathode for potassium-ion batteries based on uniform P3-type
130. Hao, J.; Xiong, K.; Zhou, J.; et al. Yolk-Shell P3-type K0.5[Mn0.85Ni0.1Co0.05]O2: a low-cost cathode for potassium-ion batteries. Energy. Environ. Mater. 2022, 5, 261-9.
131. Lv, J.; Wang, B.; Hao, J.; et al. Single-crystalline Mn-based oxide as a high-rate and long-life cathode material for potassium-ion battery. eScience 2023, 3, 100081.
132. Zhao, S.; Yan, K.; Munroe, P.; Sun, B.; Wang, G. Construction of hierarchical K1.39Mn3O6 spheres via AlF3 coating for high-performance potassium-ion batteries. Adv. Energy. Mater. 2019, 9, 1803757.
133. Weng, J.; Duan, J.; Sun, C.; et al. Construction of hierarchical K0.7Mn0.7Mg0.3O2 microparticles as high capacity & long cycle life cathode materials for low-cost potassium-ion batteries. Chem. Eng. J. 2020, 392, 123649.
134. Duan, L.; Shao, C.; Liao, J.; et al. A P2/P3 biphasic layered oxide composite as a high-energy and long-cycle-life cathode for potassium-ion batteries. Angew. Chem. Int. Ed. 2024, 63, e202400868.
135. Liu, C. L.; Luo, S. H.; Huang, H. B.; Zhai, Y. C.; Wang, Z. W. Influence of Na-substitution on the structure and electrochemical properties of layered oxides K0.67Ni0.17Co0.17Mn0.66O2 cathode materials. Electrochim. Acta. 2018, 286, 114-22.
136. Ni, L.; Xu, G.; Li, C.; Cui, G. Electrolyte formulation strategies for potassium-based batteries. Exploration 2022, 2, 20210239.
137. Wang, H.; Nie, L.; Chu, X.; et al. Flame-retardant nonaqueous electrolytes for high-safety potassium-ion batteries. Small. Methods. 2024, 8, e2301104.
138. He, G.; Nazar, L. F. Crystallite size control of Prussian white analogues for nonaqueous potassium-ion batteries. ACS. Energy. Lett. 2017, 2, 1122-7.
139. Zhao, S.; Li, G.; Zhang, B.; et al. Highly-solvating electrolyte enables mechanically stable and inorganic-rich cathode electrolyte interphase for high-performing potassium-ion batteries. Adv. Mater. 2024, 36, e2405184.
140. Liu, S.; Mao, J.; Zhang, Q.; et al. An intrinsically non-flammable electrolyte for high-performance potassium batteries. Angew. Chem. Int. Ed. 2020, 59, 3638-44.
141. Zhang, D.; Fu, H.; Ma, X.; et al. Nonflammable phosphate-based electrolyte for safe and stable potassium batteries enabled by optimized solvation effect. Angew. Chem. Int. Ed. 2024, 63, e202405153.
142. Gu, M.; Zhou, X.; Yang, Q.; et al. Anion-reinforced solvation structure enables stable operation of ether-based electrolyte in high-voltage potassium metal batteries. Angew. Chem. Int. Ed. 2024, 63, e202402946.
143. Gao, Y.; Li, W.; Ou, B.; et al. A dilute fluorinated phosphate electrolyte enables 4.9 v-class potassium ion full batteries. Adv. Funct. Mater. 2023, 33, 2305829.
144. Fan, L.; Xie, H.; Hu, Y.; et al. A tailored electrolyte for safe and durable potassium ion batteries. Energy. Environ. Sci. 2023, 16, 305-15.
145. Li, F.; Gu, X.; Cui, A.; et al. In situ structure modulation of cathode-electrolyte interphase for high-performance potassium-ion battery. Adv. Funct. Mater. 2024, 34, 2313146.
146. Zhang, H.; Wang, H.; Li, W.; et al. Enabling high-performance potassium-ion batteries by manipulating interfacial chemistry. Adv. Funct. Mater. 2024, 34, 2312368.
147. Wang, T.; He, X.; Zhou, M.; et al. In situ ions induced formation of KxF-rich SEI layers toward ultrastable life of potassium-ion batteries. Adv. Mater. 2024, 36, e2401943.
148. Hosaka, T.; Matsuyama, T.; Kubota, K.; Yasuno, S.; Komaba, S. Development of KPF6/KFSA binary-salt solutions for long-life and high-voltage K-ion batteries. ACS. Appl. Mater. Interfaces. 2020, 12, 34873-81.
149. Yang, X.; Rogach, A. L. Electrochemical techniques in battery research: a tutorial for nonelectrochemists. Adv. Energy. Mater. 2019, 9, 1900747.
150. Liu, X.; Tong, Y.; Wu, Y.; Zheng, J.; Sun, Y.; Li, H. In-depth mechanism understanding for potassium-ion batteries by electroanalytical methods and advanced in situ characterization techniques. Small. Methods. 2021, 5, e2101130.
151. Zhao, S.; Liu, Z.; Xie, G.; et al. High-efficiency cathode potassium compensation and interfacial stability improvement enabled by dipotassium squarate for potassium-ion batteries. Energy. Environ. Sci. 2022, 15, 3015-23.
152. Yu, Q.; Hu, J.; Wang, W.; et al. K0.6CoO2-xNx porous nanoframe: a co-enhanced ionic and electronic transmission for potassium ion batteries. Chem. Eng. J. 2020, 396, 125218.
153. Hwang, J. Y.; Kim, J.; Yu, T. Y.; et al. A new P2-type layered oxide cathode with superior full-cell performances for K-ion batteries. J. Mater. Chem. A. 2019, 7, 21362-70.
154. Lei, K.; Zhu, Z.; Yin, Z.; Yan, P.; Li, F.; Chen, J. Dual interphase layers in situ formed on a manganese-based oxide cathode enable stable potassium storage. Chem 2019, 5, 3220-31.
155. Zhang, Q.; Didier, C.; Pang, W. K.; et al. Structural insight into layer gliding and lattice distortion in layered manganese oxide electrodes for potassium-ion batteries. Adv. Energy. Mater. 2019, 9, 1900568.
156. Liu, L.; Liang, J.; Wang, W.; et al. A P3-type K1/2Mn5/6Mg1/12Ni1/12O2 cathode material for potassium-ion batteries with high structural reversibility secured by the Mg-Ni pinning effect. ACS. Appl. Mater. Interfaces. 2021, 13, 28369-77.
157. Liang, J.; Lin, C.; Meng, X.; et al. P3-type K0.45Co1/12Mg1/12Mn5/6O2 as a superior cathode material for potassium-ion batteries with high structural reversibility ensured by Co-Mg Co-substitution. J. Mater. Chem. A. 2021, 9, 17261-9.
158. Jo, J. H.; Choi, J. U.; Park, Y. J.; et al. P2-K0.75[Ni1/3Mn2/3]O2 cathode material for high power and long life potassium-ion batteries. Adv. Energy. Mater. 2020, 10, 1903605.
159. Jha, P. K.; Golubnichiy, A.; Sachdeva, D.; et al. Chimie douce derived novel P2-type layered oxide for potassium-ion batteries. Adv. Funct. Mater. 2024, 34, 2410665.
160. Sohn, W.; Chae, J. S.; Lim, G. H.; Roh, K. C. Ion-exchange-assisted Li0.27K0.72Ni0.6Co0.2Mn0.2O2 cathode in potassium-ion batteries. J. Alloys. Compd. 2022, 898, 162904.
161. Liu, Z. D.; Gao, X. W.; Mu, J. J.; et al. Multiphase riveting structure for high power and long lifespan potassium-ion batteries. Adv. Funct. Mater. 2024, 34, 2315006.
162. Luo, K.; Roberts, M. R.; Hao, R.; et al. Charge-compensation in 3d-transition-metal-oxide intercalation cathodes through the generation of localized electron holes on oxygen. Nat. Chem. 2016, 8, 684-91.
163. Maitra, U.; House, R. A.; Somerville, J. W.; et al. Oxygen redox chemistry without excess alkali-metal ions in Na2/3[Mg0.28Mn0.72]O2. Nat. Chem. 2018, 10, 288-95.
164. Liu, B.; Zhang, Q.; Ali, U.; et al. Solid-solution reaction suppresses the Jahn-Teller effect of potassium manganese hexacyanoferrate in potassium-ion batteries. Chem. Sci. 2022, 13, 10846-55.
165. Pandey, A. K.; Campéon, B. D.; Konuma, I.; Yabuuchi, N. P3-type layered K0.6Cr0.6Ti0.4O2 for potassium storage applications. Energy. Adv. 2023, 2, 98-102.
166. Dang, R.; Li, N.; Yang, Y.; et al. Designing advanced P3-type K0.45Ni0.1Co0.1Mn0.8O2 and improving electrochemical performance via Al/Mg doping as a new cathode material for potassium-ion batteries. J. Power. Sources. 2020, 464, 228190.
167. Zheng, Y.; Li, J.; Ji, S.; et al. Zinc-doping strategy on P2-type Mn-based layered oxide cathode for high-performance potassium-ion batteries. Small 2023, 19, e2302160.
168. Wang, H.; Meng, J.; Xiao, Z.; et al. Strain-modulated Mn-rich layered oxide enables highly stable potassium-ion batteries. Energy. Storage. Mater. 2024, 67, 103324.
169. Ai, R.; Zhang, X.; Li, S.; Wei, Z.; Chen, G.; Du, F. Selective lattice doping enables a low-cost, high-capacity and long-lasting potassium layered oxide cathode for potassium and sodium storage. Chemistry 2024, 30, e202400791.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Data & Comments
Data





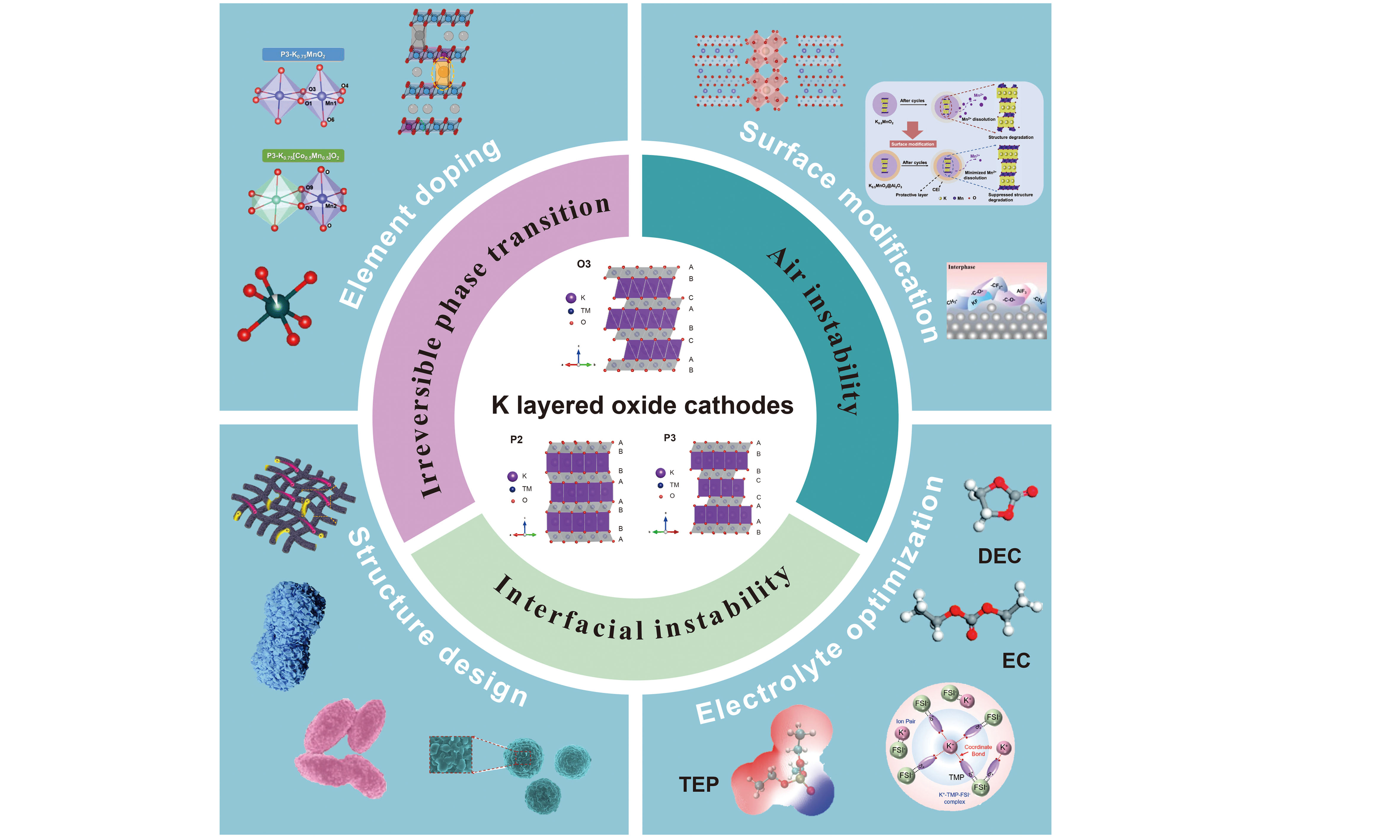
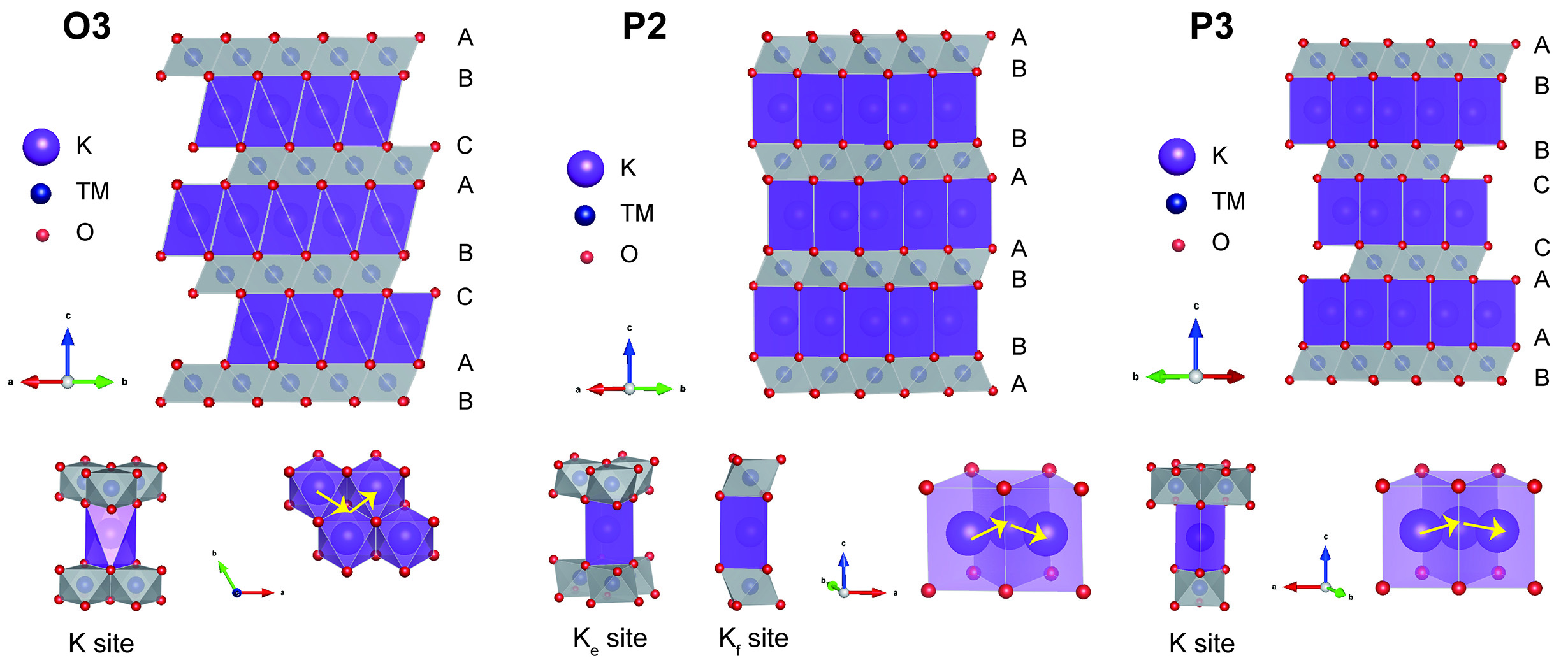
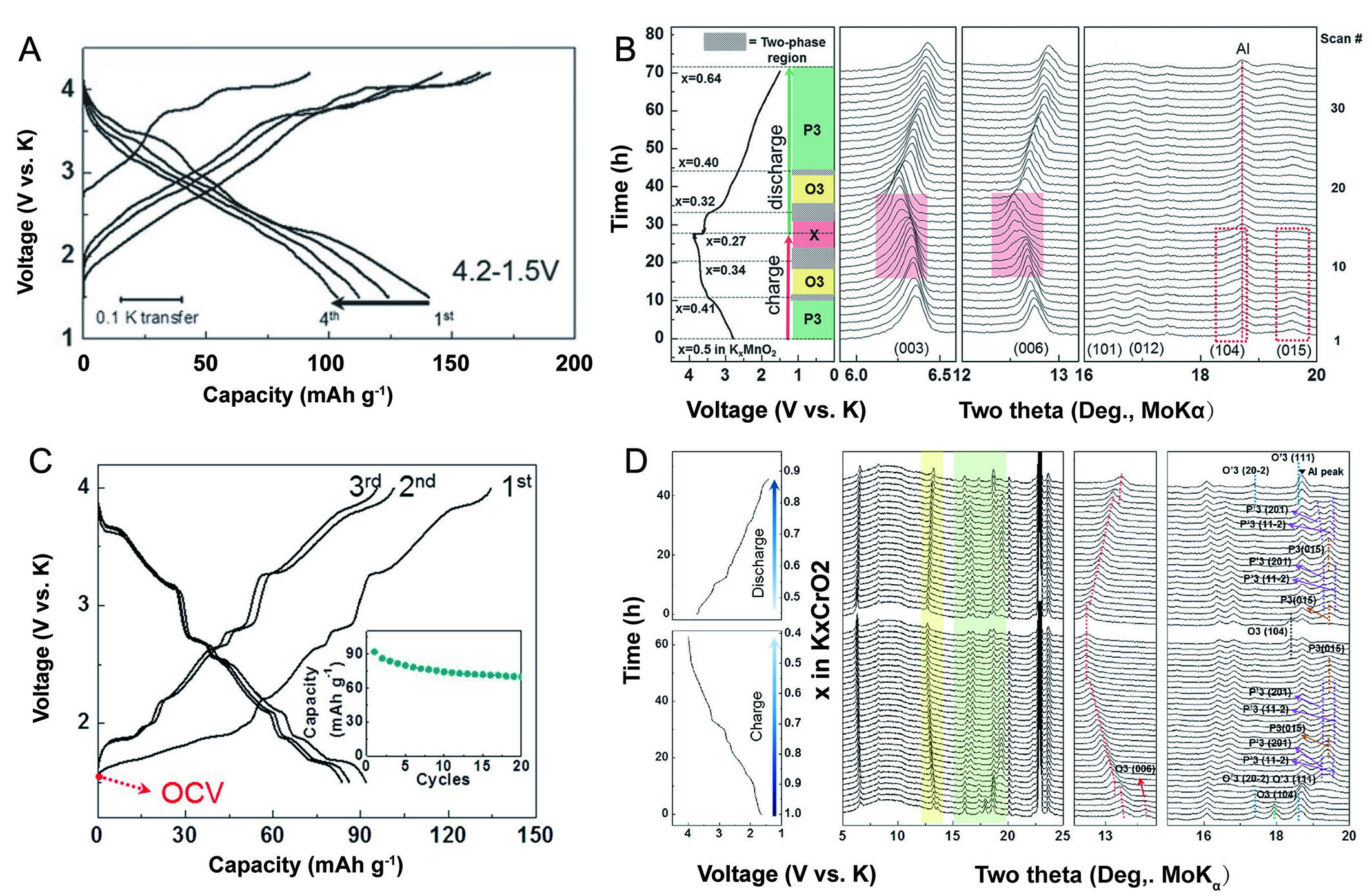
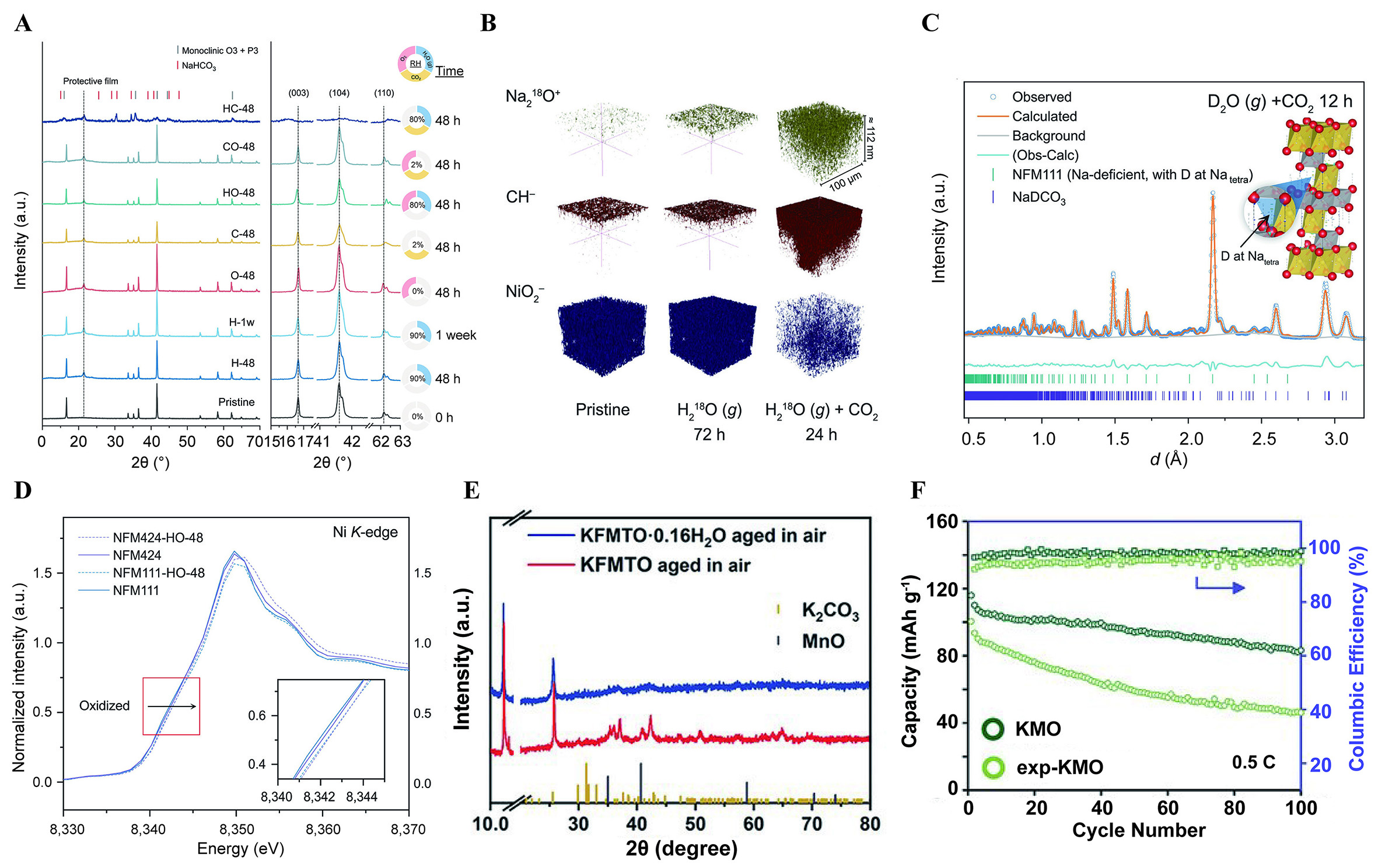
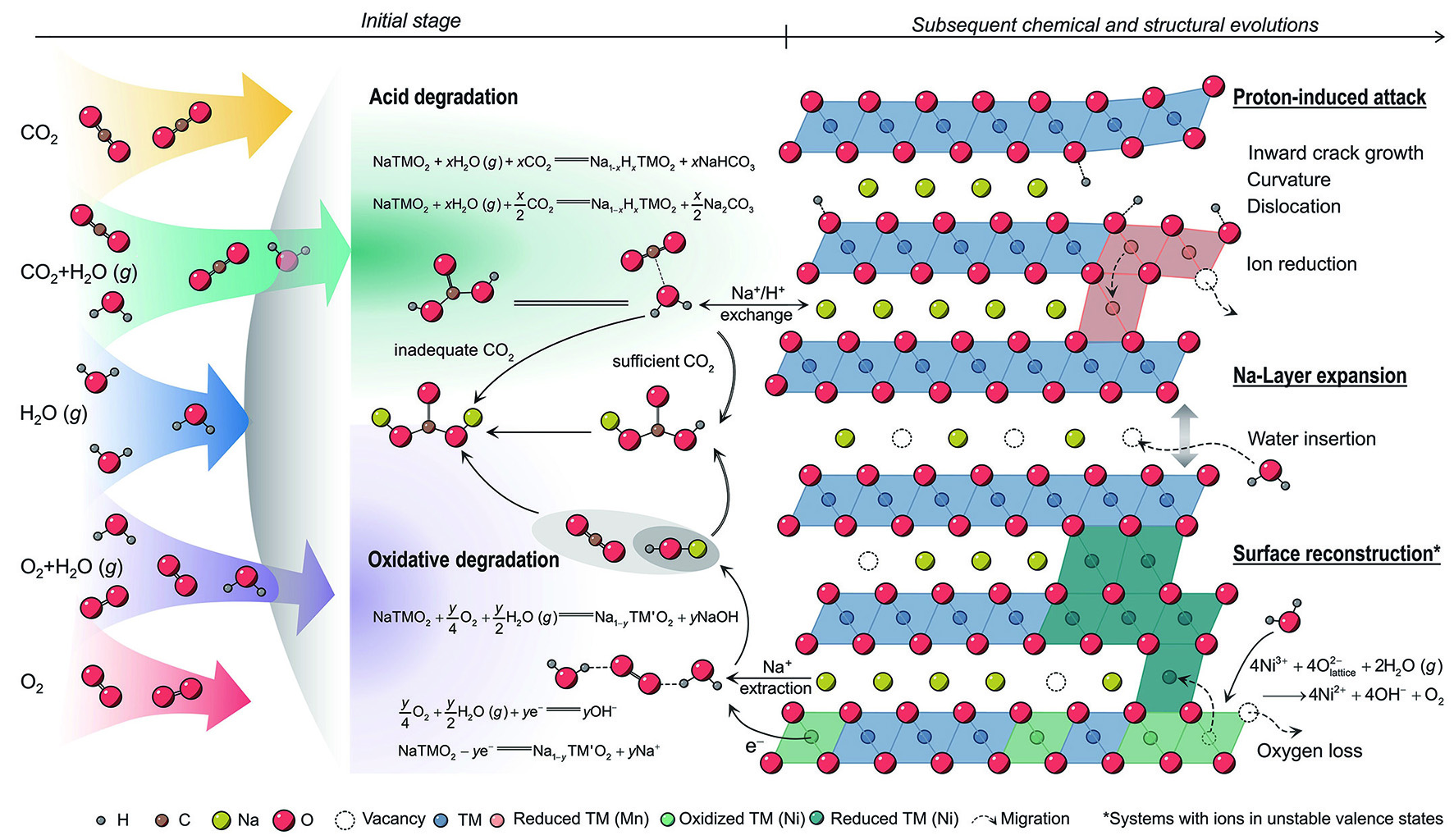
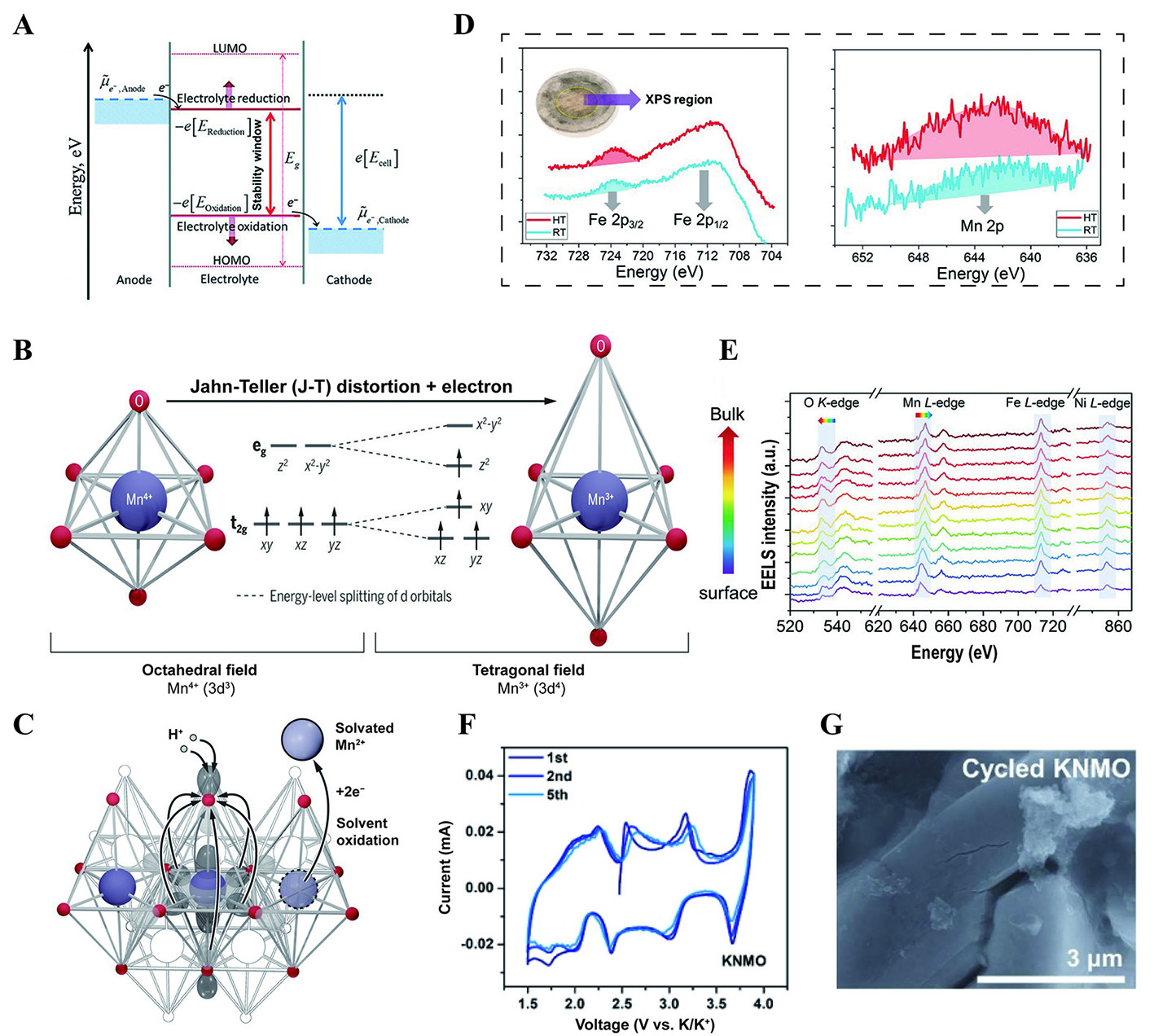
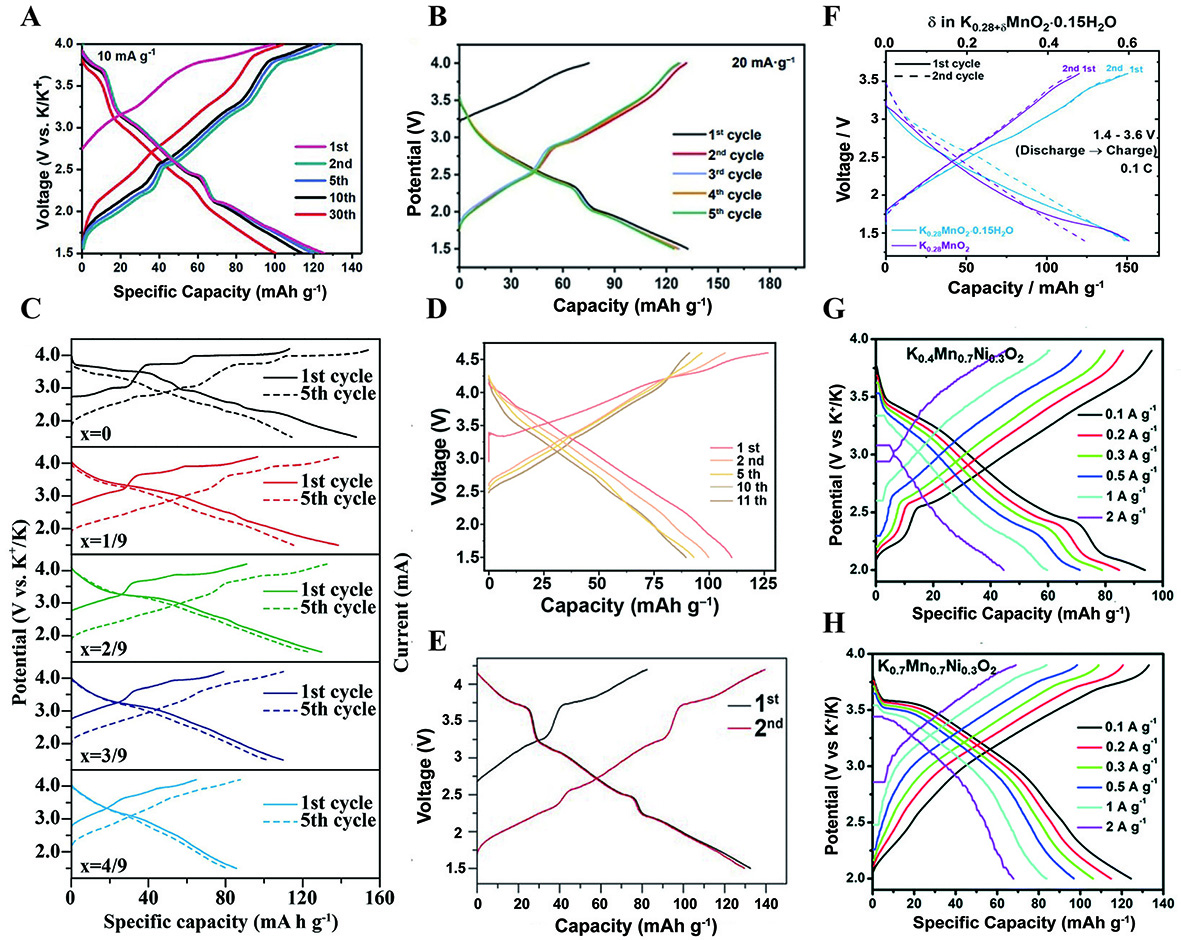
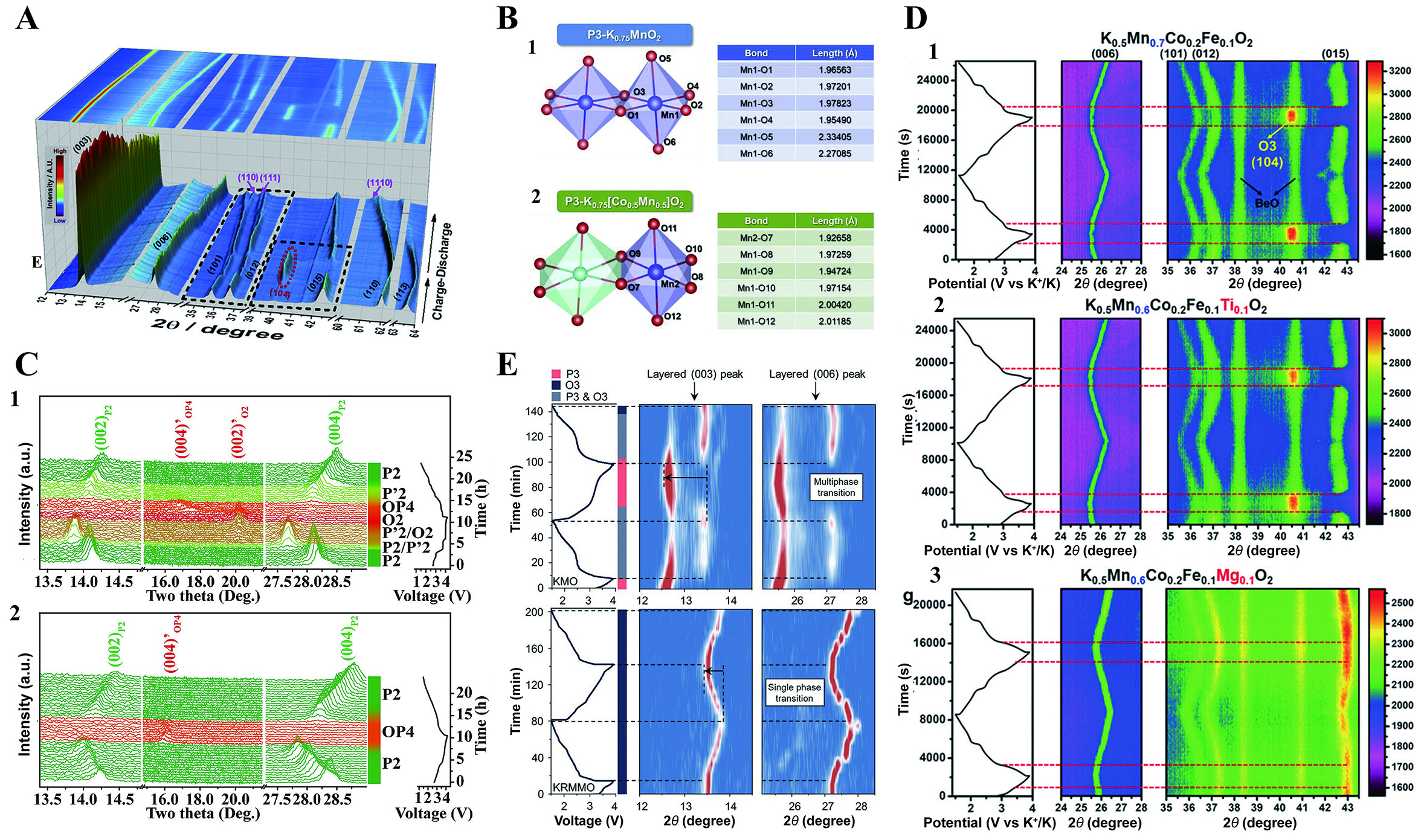
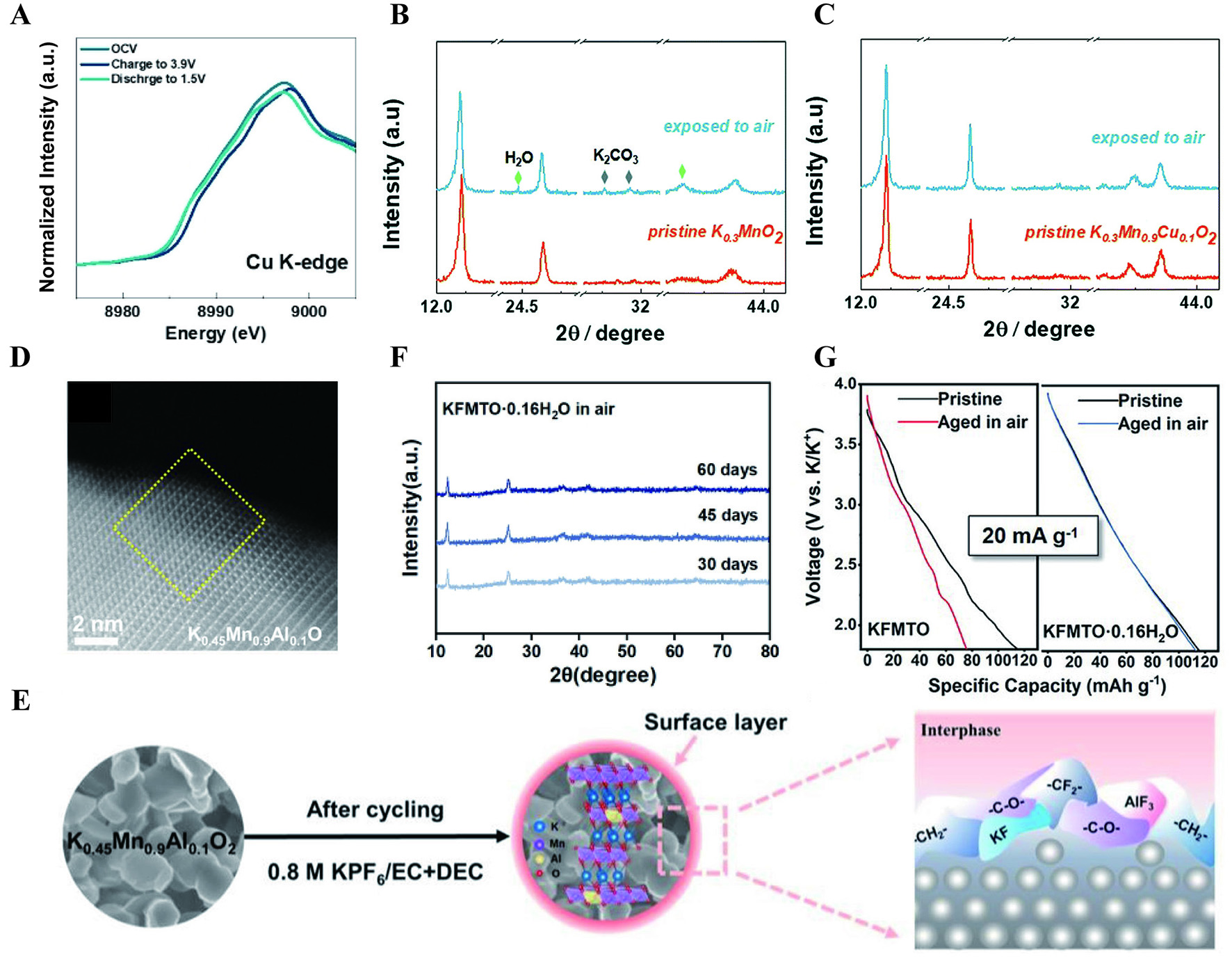
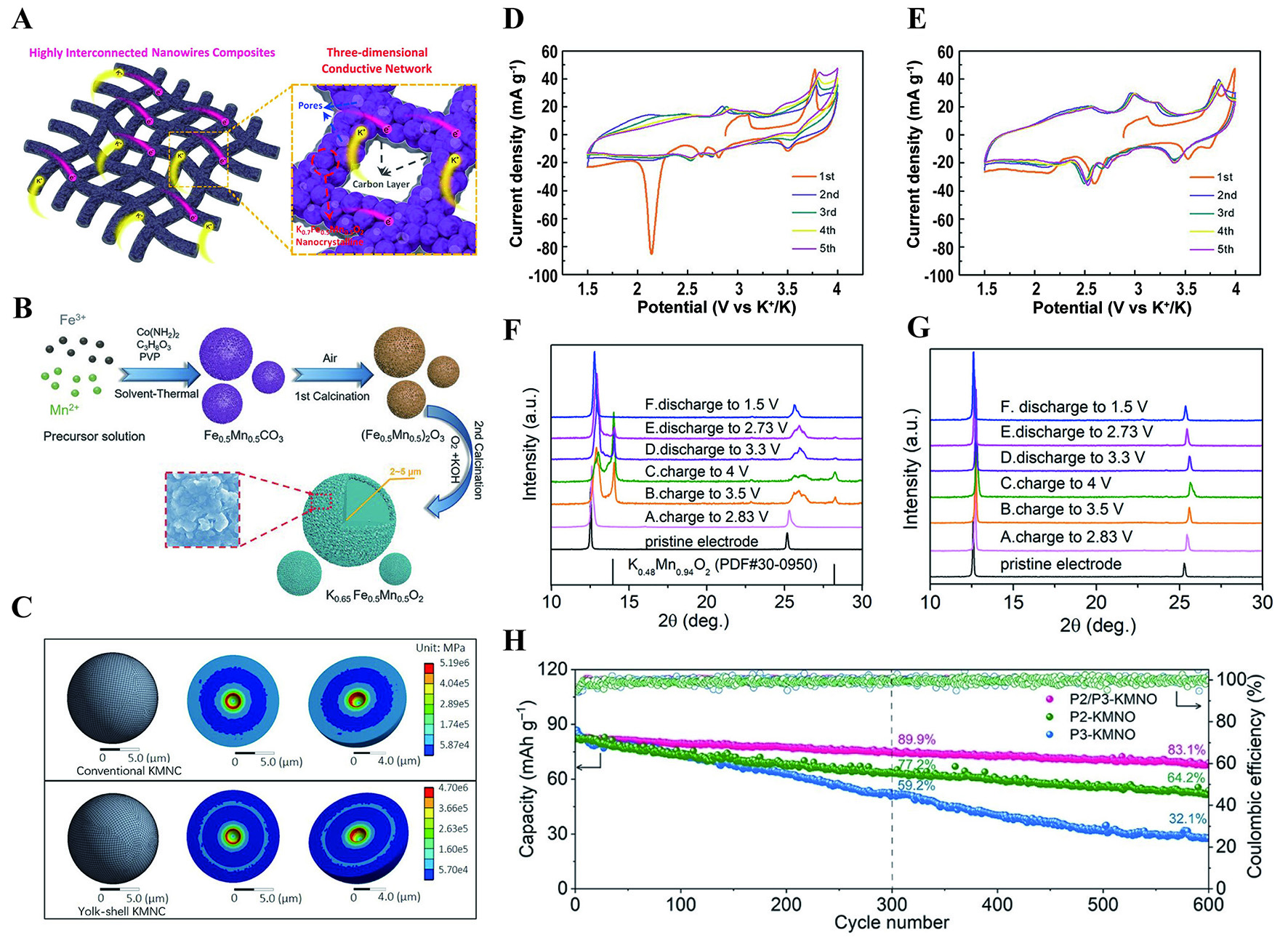
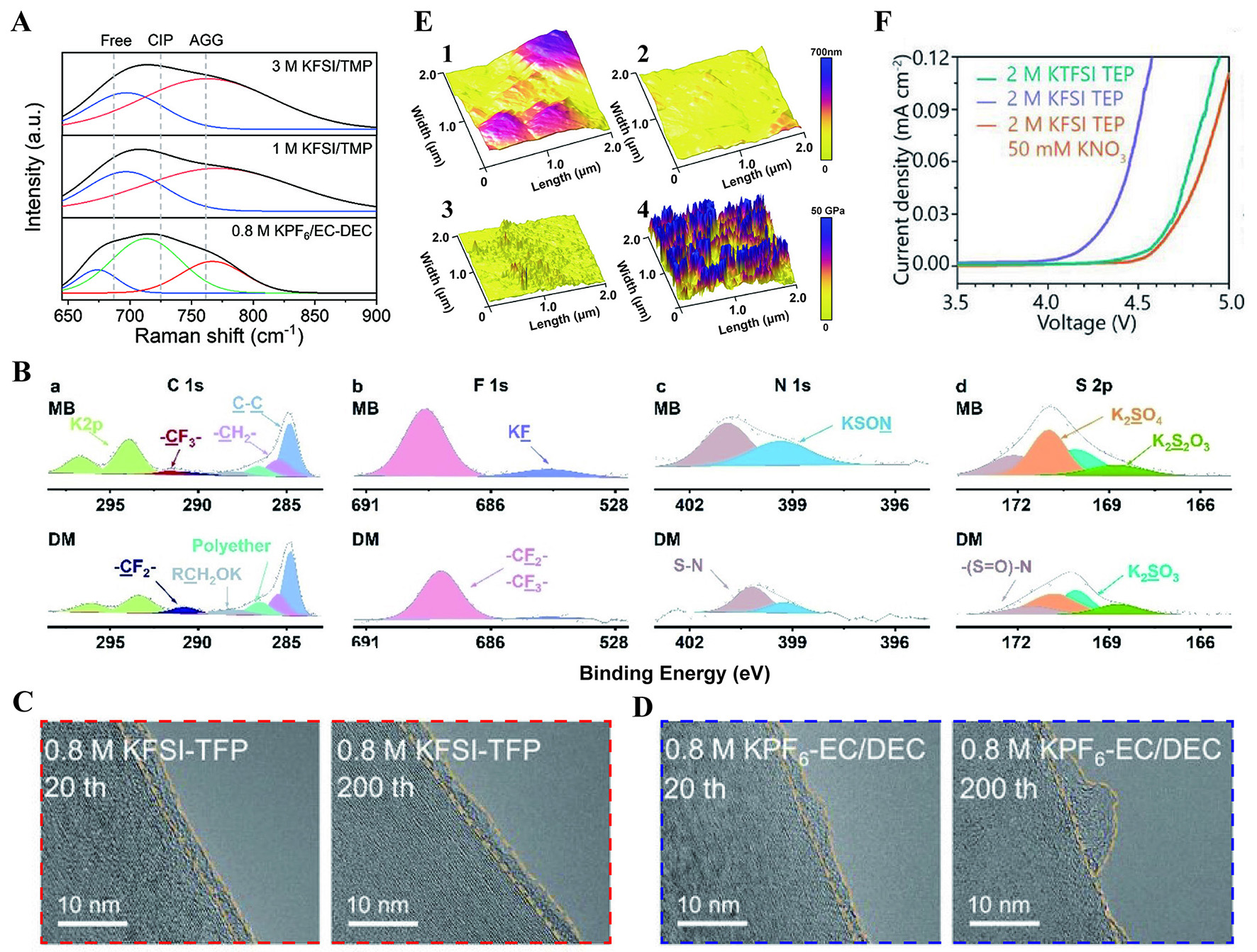












Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.