Circular RNA-based liquid biopsy: a promising approach for monitoring drug resistance in cancer
Abstract
Drug resistance remains a significant challenge in achieving successful cancer treatment, often leading to disease recurrence and reduced patient survival. While traditional tissue biopsies provide valuable insights into tumor biology, they are invasive, infrequent, and may fail to capture the full complexity of tumor heterogeneity and dynamic molecular changes. In contrast, liquid biopsy has emerged as a minimally invasive, real-time approach for monitoring tumor evolution through the analysis of circulating biomarkers. Among these biomarkers, circular RNAs (circRNAs) - a distinct class of non-coding RNAs characterized by covalently closed-loop structures - have gained attention due to their remarkable stability, abundance in body fluids, and functional involvement in gene regulation. Increasing evidence supports the role of circRNAs in mediating drug resistance through mechanisms such as inhibition of apoptosis, epithelial-mesenchymal transition, autophagy, and drug efflux, largely via interactions with microRNAs or proteins. Advanced detection methods, including quantitative reverse transcription polymerase chain reaction, droplet digital polymerase chain reaction, and RNA sequencing, combined with computational tools, enable precise profiling of circRNAs in plasma or exosomes. CircRNA-based liquid biopsies offer a dynamic, non-invasive strategy for early detection of therapeutic resistance and may guide personalized treatment decisions. This review highlights the technological advancements, biological relevance, and clinical promise of circRNAs as circulating biomarkers, emphasizing their potential in precision oncology and future collaborative translational applications.
Keywords
INTRODUCTION
The emergence of drug resistance in cancer therapy is one of the greatest obstacles to long-term therapeutic success. Even with advances in chemotherapy, targeted therapies, and immunotherapies, many solid tumors relapse in patients due to either intrinsic or acquired resistance mechanisms[1]. While biopsies are considered the gold standard for tumor characterization, tissue sampling is invasive, usually performed only once at diagnosis, and fails to capture the temporal and spatial heterogeneity of tumor biology[2]. As precision oncology advances, there is a need for dynamic and non-invasive methods to assess cancer progression and therapy response[3]. Liquid biopsy presents this opportunity by assessing tumor-derived elements [circulating tumor cells (CTCs), circulating tumor DNA (ctDNA), etc.] from biofluids, most commonly blood, urine, or saliva[4]. New markers in liquid biopsy include circular RNAs (circRNAs) and other non-coding RNAs, which have garnered attention as potential biomarkers in the context of drug resistance due to their structural stability, abundance, linearity of representation, and biologically relevant functional states[5]. CircRNAs represent a class of endogenous non-coding RNAs identified by a covalently closed-loop structure that is resistant to degradation by exonucleases[6]. Their circular structure confers exceptional stability in bodily fluids, making them promising candidates for biomarker discovery[7]. CircRNAs were initially dismissed as transcriptional noise, but they are now recognized as regulators of biological processes such as proliferation, apoptosis, autophagy, and gene expression[8]. A growing body of evidence has shown that circRNAs are involved in the development of resistance to anticancer therapies through multiple mechanisms, including sponging microRNAs (miRNAs), interacting with RNA-binding proteins (RBP), regulating signal transduction pathways, and modulating transcription[9].
The finding of circulating circRNAs through liquid biopsy represents a new frontier for non-invasive monitoring of drug resistance in a cancer context[10]. Given that circRNAs are stable in plasma and other fluids, we can readily quantify circRNA presence and abundance through innovative molecular techniques, including quantitative reverse transcription polymerase chain reaction (qRT-PCR), RNA sequencing (RNA-seq), and droplet digital PCR (ddPCR)[5]. Therefore, researchers have been able to link resistance to specific therapies with cancer-specific circRNA signatures[11]. For example, in non-small cell lung cancer (NSCLC), circRNA_102231 was shown to be overexpressed in cases where NSCLC patients had resistance to gefitinib, an epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitor (TKI)[12]. The mechanism involved acting as a sponge to miR-130a-3p, which resulted in upregulation of oncogenic miR targets. As another example, in the context of breast cancer, circRNA cerebellar degeneration-related protein 1 gene (CDR1) antisense RNA (CDR1as) was correlated with tamoxifen resistance through modulation of the miR-7/EGFR pathway[13]. Cancer drug resistance is often multifaceted and involves changes in drug metabolism, expression of efflux pumps, DNA repair, epithelial-mesenchymal transition (EMT), and stemness. CircRNAs have the potential to disrupt many of these pathways[14]. For example, circHIPK3 has been shown to support chemoresistance by targeting various downstream processes in colorectal and bladder cancers[15]. The involvement of circRNAs in modulating resistance at multiple levels highlights their promise as unifying biomarkers that profile the changing resistance landscape during treatment[16]. In addition, it is plausible that circRNAs may provide a better biological picture of tumor heterogeneity than a biopsy from only one tumor site, given that circRNAs may be from diverse tumor sites[17]. Despite the very optimistic possibilities, challenges remain in circulating circRNAs for clinical purposes[18]. First, protocols for sample collection, RNA isolation, and data normalization must be established to ascertain reproducibility. Second, there may be detection and validation issues with lowly abundant circRNAs[19]; circumvention of low amounts of rare circRNAs may require a very sensitive detection method[20]. A third significant challenge is to reliably differentiate tumor-derived circRNAs from circRNAs in normal tissues[21]. Fourth, circRNAs with significant clinical value must be further validated in extensive, multi-center translational studies and matched to numerous cancer types and treatment approaches[22]. The detection of circulating circRNAs using liquid biopsy represents a watershed innovation in cancer diagnostics and therapeutic monitoring[22]. Real-time detection of molecular underpinnings of drug resistance would enhance personalized treatment through circRNA profiling to guide drug choice and ultimately improve patient outcomes[23]. As this exciting field develops further, circRNA biomarkers are expected to be integrated into clinical practice, facilitating more adaptive cancer care within the context of personalized medicine.
CIRCRNAS FOR TRACKING DRUG RESISTANCE IN CANCER
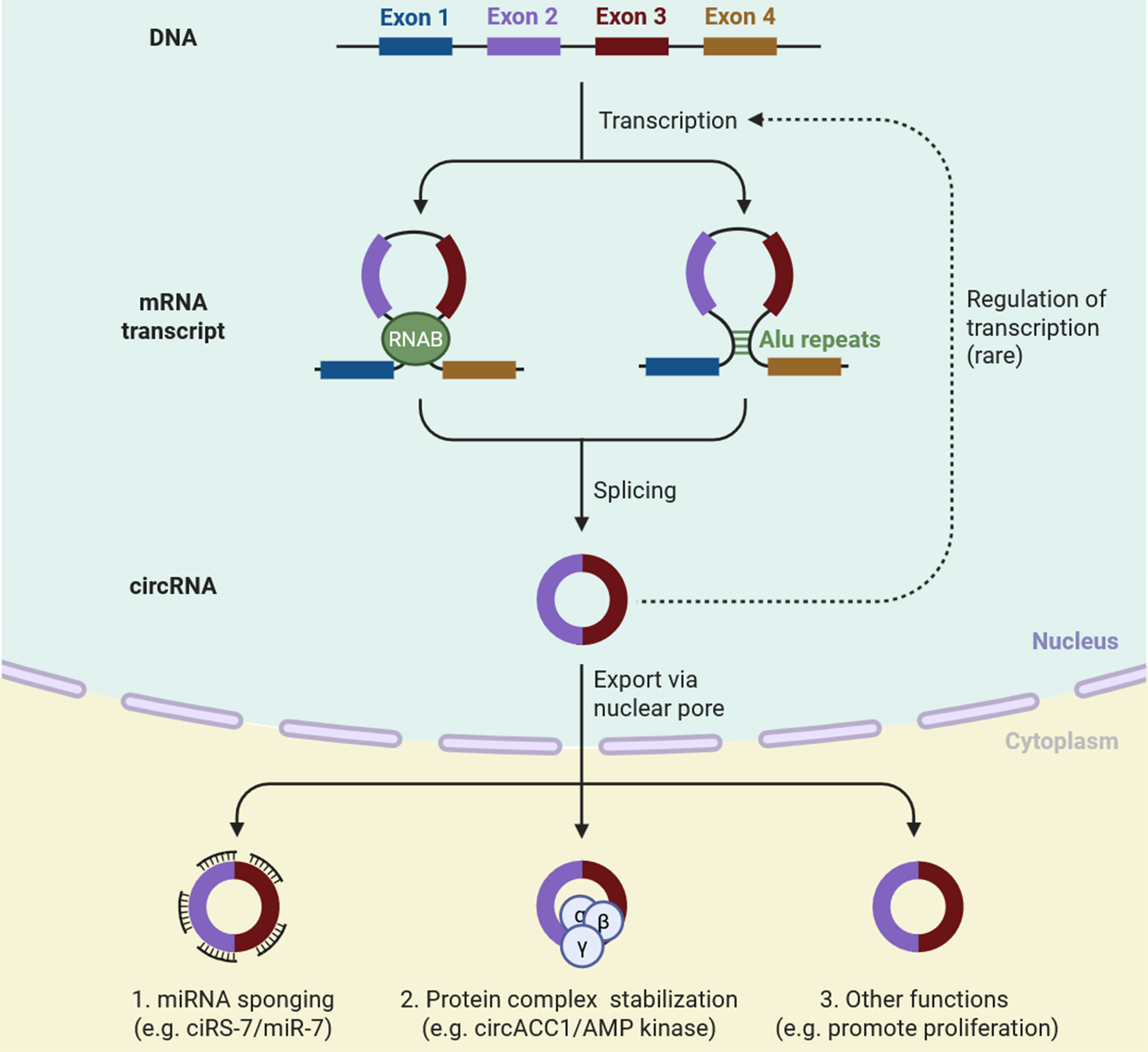
CircRNAs are generated from pre-mRNA transcripts through a unique phenomenon known as back-splicing. Back-splicing is an event where a downstream splice donor connects to an upstream splice acceptor[24]. Back-splicing can occur with the help and support of RBP or inverted repeat elements - sequences that bring exons into close proximity, enabling their circularization[25]. The resulting circRNAs consist of covalently closed loop structures and therefore lack 5′ caps or 3′ poly (A) tails[26]. The absence of these terminal modifications contributes to the very high stability of circRNAs compared with linear RNAs [Figure 1][27]. CircRNAs can additionally stabilize protein complexes, as demonstrated by circACC1, which aids in the assembly of the adenosine monophosphate (AMP) kinase complex and has a role in metabolism[28]. CircRNAs have also been associated with promoting proliferation, survival, and resistance to therapy in cancer cells by interacting with major signal transduction pathways[29]. They are not commonly involved in the regulation of transcription in the nucleus; however, there is an emerging role (either positive or negative) for some circRNAs to direct gene expression in multiple ways
Figure 1. This schematic illustrates the formation of circRNAs through back-splicing of pre-mRNA, where exons are circularized via RBP or Alu repeat elements. The resulting circRNA is exported from the nucleus into the cytoplasm. CircRNAs can perform multiple regulatory functions, including (1) miRNA sponging (e.g., ciRS-7 sequestering miR-7); (2) stabilization of protein complexes (e.g., circACC1 supporting AMP kinase activity); and (3) other cellular roles, such as promoting proliferation and modulating gene expression. Though rare, circRNAs may also influence transcriptional regulation in the nucleus. These diverse functions contribute to circRNAs’ roles in cancer progression and drug resistance [Created in BioRender. Singh DD (2025)]. circRNAs: Circular RNAs; RBP: RNA-binding proteins; miRNA: microRNA; AMP: adenosine monophosphate.
Key circRNAs implicated in cancer drug resistance: pathways, mechanisms, and clinical applications
| S.N. | CircRNA name | Tumor type | Target pathway/gene | Mechanism of drug resistance | Clinical relevance/potential use | Ref. |
| 1 | circHIPK3 | Colorectal, lung, bladder | miR-124, miR-558 | Acts as a sponge to suppress tumor-suppressor miRNAs; promotes resistance to 5-FU and cisplatin | Biomarker for chemotherapy resistance | [33] |
| 2 | circFOXO3 | Breast, lung, gastric | FOXO3, p21, CDK2 | Interferes with cell cycle regulation and apoptosis pathways | Prognostic marker; potential therapeutic target | [38] |
| 3 | circRNA_100290 | Oral squamous cell carcinoma | miR-29 family | Modulates cell proliferation and cisplatin resistance | Diagnostic and drug response predictor | [39] |
| 4 | circ_0001946 | NSCLC | miR-135a-5p, STAT6 | Promotes gefitinib resistance by activating STAT6/PI3K/AKT pathway | Potential marker for EGFR-TKI resistance monitoring | [40] |
| 5 | circRNA CDR1as | Glioma, breast | miR-7, EGFR pathway | Regulates drug response via miRNA sponging and EGFR signaling | Associated with resistance to targeted therapy | [41] |
| 6 | circ-PVT1 | Gastric cancer | miR-124-3p, ZEB1 | Facilitates paclitaxel resistance by modulating EMT | Marker of chemoresistance and poor prognosis | [36] |
| 7 | circMTO1 | HCC | miR-9/p21 | Enhances doxorubicin sensitivity via tumor suppressor pathways | Therapeutic sensitization target | [42] |
| 8 | circAKT3 | GBM | PI3K/AKT pathway | Promotes TMZ resistance via maintaining stemness | Candidate for targeting glioma stem cells | [43] |
| 9 | circ-ABCB10 | Breast, lung cancer | miR-1271, BCL2 | Enhances resistance by modulating apoptosis and cell survival | Liquid biopsy candidate for resistance monitoring | [29] |
LIQUID BIOPSY-BASED DETECTION OF CIRCULATING CIRCRNAS
Cancer treatment has evolved considerably with the introduction of targeted therapies, immunotherapies, and combination regimens[29]. Unfortunately, drug resistance (whether intrinsic or acquired) remains a significant challenge that can culminate in therapeutic failure, progression of disease, and suboptimal survival[44]. Due to the dynamic and heterogeneous nature of tumors, it is wise to continually assess molecular changes during treatment[6]. Although tissue biopsies can be educational, they are invasive, typically only provide a limited evaluation of tumor biology, and cannot be used for longitudinal monitoring[45]. As a result, liquid biopsy has emerged as a novel, non-invasive application to assess real-time cancer progression and treatment resistance[46]. Table 2 presents a comparative analysis of the components involved in liquid biopsy-based detection of circulating circRNAs for monitoring drug resistance in cancer[55]. Liquid biopsy is the characterization of tumor-derived constituents, such as CTCs, ctDNA, extracellular vesicles, and different species of RNA, including circRNAs, in biofluids (e.g., blood)[56]. Due to their closed-loop structure that lacks free 5′ and 3′ ends, circRNAs resist exonuclease degradation, enabling them to freely circulate and serve as stable and strong biomarkers[6]. In the cancer context, circRNAs can exhibit multiple roles, e.g., serving as a miRNA sponge, interacting with RBP, regulating transcription, and even translated into functional peptides[16,57]. Importantly, dysregulated circRNA expression could result in mechanisms of drug resistance across multiple malignancies, such as lung, breast, liver, and colon cancer[44]. CircRNAs detected through liquid biopsy will have a multitude of advantages for the clinic, many based on what we discussed in the previous sections[24]. First, circRNAs in liquid biopsies are exceptionally stable, allowing their detection in biofluids often without immediate specimen processing[58]. Second, liquid biopsies enable clinicians to collect real-time samples from patients multiple times, providing insights into system or tumor responses and facilitating early detection of resistance[2]. Third, liquid biopsies are non-invasive and can obtain biospecimens from patients who might otherwise be ineligible due to poor general condition, prior trauma, or advanced disease[59]. Fourth, circulating circRNAs can originate from multiple tumor sites, offering a more comprehensive view of the disease and potentially revealing emerging resistance profiles simultaneously[59,60]. Recent advancements in technology have enabled sensitive and specific detection of circRNAs from plasma and serum samples using qRT-PCR, ddPCR and RNA-seq methods[60]. These platforms allow for profiling circRNA expression changes during treatment, which may provide early signals of therapeutic failure or developing resistance. Numerous preclinical and clinical studies have identified circRNA signatures correlated with chemoresistance, resistance to tyrosine kinase inhibitors, and resistance to immune checkpoint blockade, highlighting their translational potential[61]. Despite these promising advancements, challenges exist. There remains a lack of standardization for the many pre-analysis variables that can influence circRNA measurements (e.g., sample processing in clinical laboratories, RNA isolation protocols, and data normalization)[62]. Additionally, further validation through large, multi-center clinical studies is required to assess the diagnostic or prognostic value of specific circRNAs. Combining circRNA biomarkers with other molecular and clinical measures may enhance their predictive power and increase their utility in personalized medicine[63]. In summary, the liquid biopsy-based detection of circulating circRNAs provides a new, effective paradigm for non-invasive tracking of drug resistance in cancer[6]. As research continues and the technology advances, it is possible that circRNA profiling will become a relevant component of the clinical oncology arsenal, enabling real-time treatment guidance for drug resistance and improving outcomes in the era of precision oncology[44].
Components of liquid biopsy-based detection of circulating circRNAs for drug resistance monitoring in cancer: a comparative analysis
| S.N. | Component category | Specific component | Description/principle | Advantages | Limitations/disadvantages | Ref. |
| 1 | Biological sample | Plasma/serum | Primary source of circulating circRNAs; obtained from peripheral blood | Minimally invasive; widely used; good RNA stability | Low RNA yield; variability depending on handling | [5] |
| 2 | Biological sample | Urine/saliva/CSF | Alternative fluids for site-specific cancers (e.g., urological, CNS) | Non-invasive; can reflect local tumor biology | Lower circRNA concentrations; limited standard protocols | [6] |
| 3 | Cellular/molecular assay | Exosome isolation | Isolation of tumor-derived exosomes containing circRNAs | Enhances tumor specificity; protects RNA from degradation | Time-consuming; requires specialized kits or equipment | [47] |
| 4 | Cellular/molecular assay | RNase R treatment | Digests linear RNAs to enrich circRNAs | Increases specificity for circRNAs | May result in partial RNA loss if not optimized | [35] |
| 5 | RNA extraction method | Column-based/magnetic bead kits | Commercial kits (e.g., Qiagen, Norgen) used for isolating total RNA from biofluids | High RNA purity; optimized for low-input samples | Costly; requires careful sample handling | [48] |
| 6 | Detection principle | qRT-PCR with divergent primers | Amplifies the back-splice junction unique to circRNAs | Cost-effective; highly specific for known circRNAs | Limited to known targets; less sensitive for low-abundance circRNAs | [49] |
| 7 | Detection principle | ddPCR | Uses microdroplets to detect and quantify RNA copies with high sensitivity | Absolute quantification; ideal for rare circRNAs | Expensive; requires specialized instruments | [50] |
| 8 | Detection principle | RNA-seq | Unbiased sequencing to detect both known and novel circRNAs | Comprehensive; detects novel circRNAs and expression changes | Data-intensive; needs bioinformatics expertise | [51] |
| 9 | Bioinformatics analysis | CIRCexplorer2, find_circ, CIRI2, DCC | Tools used to identify circRNAs from sequencing data by mapping back-splice junctions | Enables genome-wide circRNA discovery | Complex pipelines; prone to false positives without proper filtering | [52] |
| 10 | Bioinformatics analysis | circBase, CircInteractome, circAtlas | Databases for annotation, interaction prediction, and biological interpretation of circRNAs | Provides functional context and regulatory network insights | Limited clinical annotations for novel circRNAs | [53] |
| 11 | Clinical relevance | Resistance monitoring | Real-time, longitudinal assessment of treatment response via circRNA dynamics | Guides therapy decisions; non-invasive surveillance | Not yet standardized for clinical use; requires large-scale validation | [45] |
| 12 | Clinical relevance | Personalized treatment adaptation | Using circRNA expression changes to tailor treatment regimens dynamically | Supports precision oncology | Translational challenges; regulatory hurdles | [54] |
CIRCULATING CIRCRNAS AS EMERGING BIOMARKERS IN LIQUID BIOPSY FOR DRUG RESISTANCE SURVEILLANCE IN CANCER
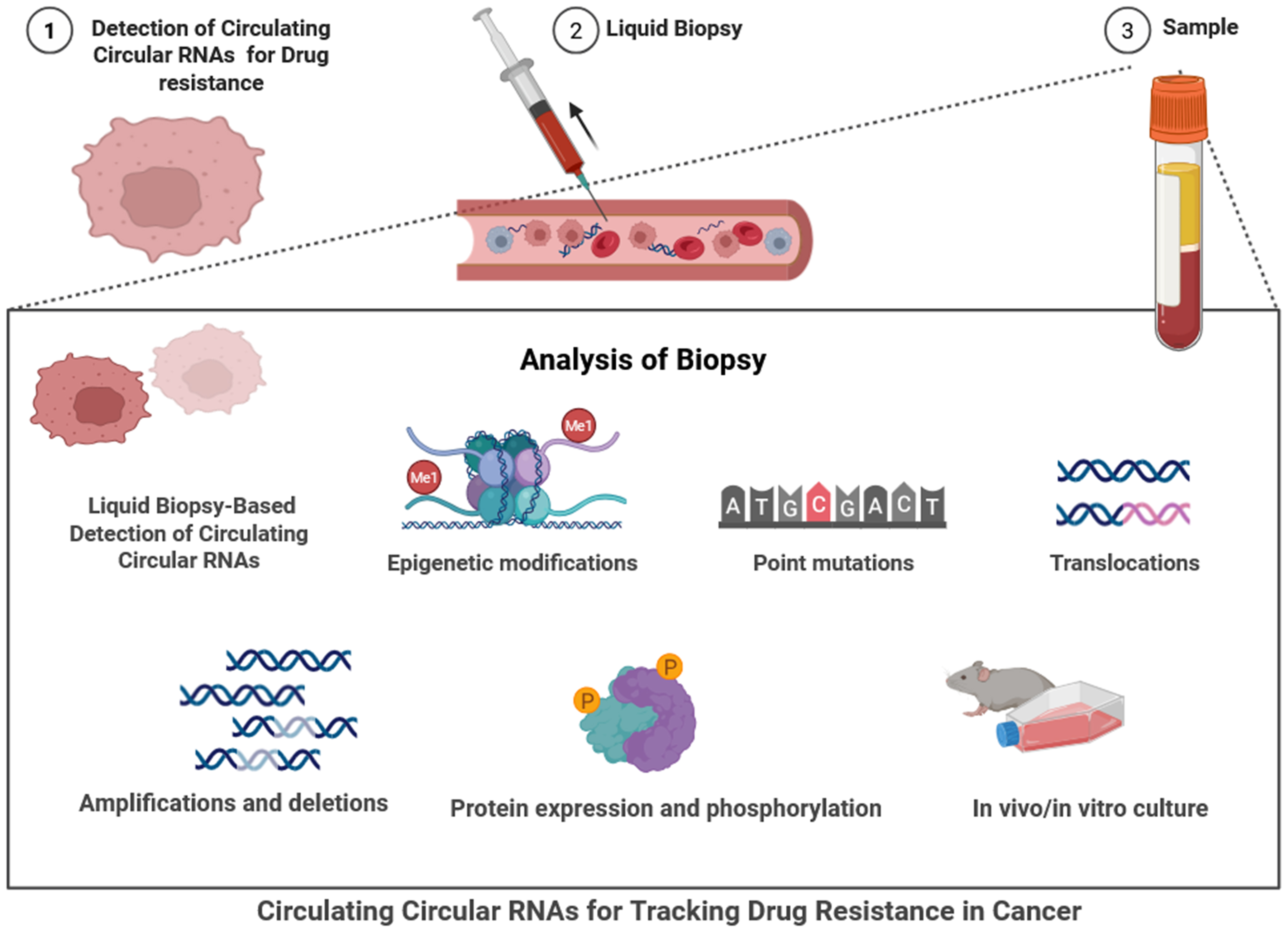
CircRNAs are becoming increasingly popular as circulating biomarkers. Evidence of their dysregulation in numerous cancers adds to our knowledge of resistance mechanisms and a bright future for therapeutic monitoring[15]. The liquid biopsy-based approach for detecting circulating circRNAs is used to monitor drug resistance in cancer[15]. The process begins with the identification of resistant cancer cells and the collection of a blood sample, from which plasma or serum is isolated[18]. Biopsy analysis involves detecting epigenetic modifications, point mutations, translocations, amplifications, deletions, and assessing protein expression and phosphorylation [Figure 2][64]. Functional assays using in vitro or in vivo models are employed to further validate the findings. Central to this approach is the detection of circRNAs, which act as stable, non-invasive biomarkers for tracking therapeutic resistance and guiding personalized cancer treatment decisions[65]. In NSCLC, the circRNA hsa_circ_0000190 and hsa_circ_0014235 were found to be upregulated in the plasma and sponge miRNAs, such as miR-142 and miR-124-3p, which promotes resistance to EGFR tyrosine kinase inhibitors[65]. In triple-negative breast cancer (TNBC), the circANKS1B found in serum contributed to the enhancement of EMT and paclitaxel resistance by targeting miR-148a-3p[66]. Likewise, circ_0005963 in plasma from colorectal cancer (CRC) enhances oxaliplatin resistance by governing glycolytic regulation through the GLUT1/miR-122 axis[67]. CircAKT3 is associated with gastric cancer targets and upregulates the autophagy pathway [PI3K/AKT/mechanistic target of rapamycin (mTOR)], helping promote cisplatin resistance[68]. In HCC, circMTO1 serves as a tumor suppressor but is downregulated in sorafenib resistance[69]. GBM-associated circRNAs, including circHIPK3 and circNT5E, have been detected in plasma and cerebrospinal fluid (CSF), with alterations in miRNA influencing TMZ resistance[70]. Similarly, circRNAs in ovarian (circCELSR1), pancreatic (circ-LDLRAD3), and bladder cancer (circRIP2) regulate pathways associated with chemotherapy resistance, including the EMT, PI3K/AKT, and transforming growth factor beta
Figure 2. The figure depicts the use of liquid biopsy-based detection of circulating circRNAs to track drug resistance in cancer, beginning with the identification of drug-resistant tumor cells (Step 1) and progressing to liquid biopsy collection via blood draw and non-invasive material (Step 2). The resulting sample (Step 3), generally plasma or serum, will undergo analysis for molecular changes associated with the resistance. The analysis options include various molecular methods to detect epigenetic modifications, point mutations, translocations, and copy number changes such as amplifications or deletions. Other assessments also include protein expression and phosphorylated proteins, as well as the use of in vivo/in vitro methodologies to establish functional changes. Importantly, the aim is to detect and characterize the stable, circulating circRNAs in the liquid biopsy, where they may serve as potential circulating biomarkers. The proposed workflow enables real-time, non-invasive capture of valuable information regarding therapeutic resistance and supports the implementation of precision oncology and therapies for cancer treatment [Created in BioRender. Singh DD (2025)]. circRNAs: Circular RNAs.
Components of liquid biopsy-based detection of circulating circRNAs for drug resistance monitoring in cancer
| S.N. | Cancer type | Liquid biomarker (circRNA) | Origin (body fluid) | Tendency in resistance | Target/pathway | Function/role | Ref. |
| 1 | NSCLC | hsa_circ_0000190, hsa_circ_0014235 | Plasma | Upregulated | miR-142, miR-124-3p | Promotes EGFR-TKI resistance by sponging miRNAs | [72] |
| 2 | TNBC | circ_0006528, circANKS1B | Serum | Upregulated | miR-148a-3p, miR-149-5p | Enhances EMT, metastasis and paclitaxel resistance | [73] |
| 3 | CRC | circ_0005963 | Plasma | Upregulated | miR-122, GLUT1 | Facilitates glycolysis and oxaliplatin resistance | [74] |
| 4 | Gastric cancer | circAKT3, circ_0000267 | Plasma | Upregulated | PI3K/AKT/mTOR | Regulates cisplatin resistance via autophagy control | [75] |
| 5 | HCC | circ_0004913, circMTO1 | Serum | Downregulated (in some) | miR-9, p21 | Acts as tumor suppressor; associated with sorafenib resistance | [76] |
| 6 | Prostate cancer | circFOXO3 | Plasma | Dysregulated | FOXO3 pathway | Modulates androgen deprivation therapy resistance | [77] |
| 7 | Pancreatic cancer | circ-LDLRAD3 | Plasma | Upregulated | miR-137 | Promotes gemcitabine resistance via EMT regulation | [78] |
| 8 | GBM | circHIPK3, circNT5E | CSF/plasma | Upregulated | miR-124, miR-422a | Enhances proliferation and TMZ resistance | [79] |
| 9 | Ovarian cancer | circCELSR1 | Plasma | Upregulated | miR-1252, FOXR2 | Contributes to cisplatin resistance via PI3K/AKT activation | [80] |
| 10 | Bladder cancer | circRIP2 | Urine/plasma | Upregulated | miR-1305, TGF-β2 | Facilitates EMT and chemoresistance | [81] |
Comparison of liquid biopsy markers for drug resistance surveillance in cancer
| S.N. | Marker type | Biological source | Detection of circRNAs | Utility in drug resistance monitoring | Advantages | Limitations | Ref. |
| 1 | ctDNA | Plasma/serum | Not applicable (DNA only) | Detects resistance mutations (e.g., EGFR, KRAS) | Non-invasive; mutation-specific tracking; quick turnaround | Does not reflect non-coding RNA regulation; low abundance in early-stage disease | [92] |
| 2 | CTCs | Whole blood | Possible (but rarely used for circRNA) | Phenotypic and genotypic profiling of resistance-related pathways | Can be cultured; provides both RNA and protein information | Low frequency; difficult to isolate and purify; not ideal for circRNA analysis | [86] |
| 3 | Exosomes | Plasma, serum, saliva, urine, CSF | Highly enriched for circRNAs | Reflects tumor-secreted resistance-related molecules and intercellular signals | Protect circRNAs from degradation; tumor-specific; suitable for dynamic tracking | Isolation and characterization methods not yet standardized; requires optimization | [91] |
| 4 | cfRNA | Plasma/serum | Includes circRNAs (after enrichment) | Tracks therapy-related changes in gene expression and resistance regulation | Minimally invasive; suitable for repeated monitoring | CircRNAs are a minor fraction of total cfRNA; requires enrichment and sensitive detection | [93] |
| 5 | Exosomal circRNAs | Exosome fraction from plasma/serum | Direct and stable source of circRNAs | Dynamic monitoring of resistance; potential predictive biomarker | High specificity and stability; resistant to RNases | Requires high-quality isolation; functional validation needed | [94] |
TOOLS AND TECHNIQUES FOR MONITORING LIQUID BIOPSY OF CIRCRNAS IN CANCER THERAPY RESISTANCE
Monitoring of circulating circRNAs by liquid biopsy utilizes various RNA detection methods, including qRT-PCR, ddPCR, and RNA-seq[94,95]. qRT-PCR is a sensitive, rapid technique, ddPCR enables absolute quantification, and RNA-seq provides comprehensive circRNA profiling. These methods differ in cost, throughput, and suitability for clinical application[96]. Non-invasive monitoring of circRNAs in circulation to track mechanisms of therapeutic resistance requires a multifaceted approach combining molecular techniques with bioinformatics methods to provide real-time insight into the biology of tumors[97,98]. Blood should be collected using RNase-free ethylenediaminetetraacetic acid (EDTA) tubes and processed quickly by centrifugation to provide plasma. Exosome preparations, whether obtained by ultracentrifugation or polystyrene-based commercial systems, can provide tumor-specific circRNAs, further increasing their utility for monitoring tumor evolution in the context of therapy[99,100]. High-sensitivity kits provide total RNA extraction that can be treated with RNase R to enrich circRNAs by removing linear RNA[101]. Finally, divergent primers can be developed for the qRT-PCR method to detect known circRNAs that can be isolated with ddPCR[102]. High-sensitivity droplet dPCR provides sensitive absolute quantitation of circRNAs as a complementary method for potentially longitudinal monitoring and/or resistance contributions[103].
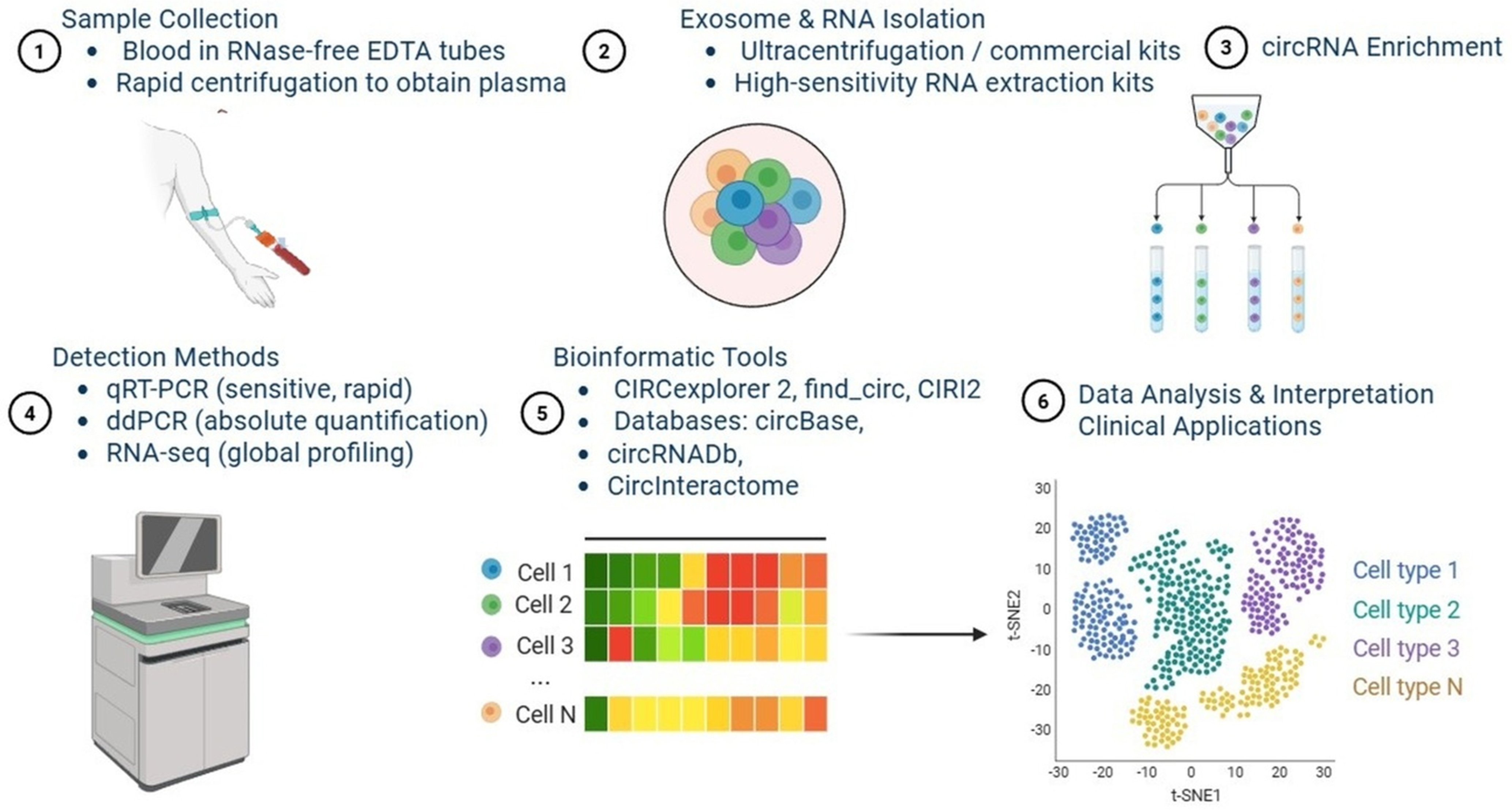
The combination of circRNA-specific back-splice junction detection with deconvolution-based methods improves specificity, which would enable the identification of cancer type- or drug resistance-associated circRNAs at low abundance. Deconvoluted sequencing in combination with longitudinal RNA-seq also facilitates early detection of therapeutic resistance[101]. Future integration of deconvoluted sequencing with artificial intelligence (AI) and single-cell RNA-seq libraries is expected to further improve sensitivity and predictive power, and to support the use of bulk RNA-seq as a non-invasive monitoring tool[101-104]. As RNA-seq becomes more affordable, global profiling of circRNAs may be achieved through the use of rRNA-depleted or RNase R-treated libraries and bioinformatics tools such as CIRCexplorer2, find_circ and CIRI2[105]. Exosome-derived circRNAs also display tumor specificity, as drug-resistant tumor cells tend to preferentially package them, which could provide insight into the persistence and localization of drug resistance during therapy[106]. The integration of bioinformatics platforms and databases (circBase, circRNADb, and CircInteractome) will facilitate circRNA annotation, differential expression analysis, and functional characterization, while incorporating pathway and network analysis platforms will help elucidate the mechanisms by which circRNAs contribute to cancer drug resistance [Figure 3][107].
Figure 3. Schematic representation of the experimental and analytical pipeline for circRNA detection and characterization. (1) Sample collection: blood is collected in RNase-free EDTA tubes, followed by rapid centrifugation to obtain plasma; (2) Exosome and RNA isolation: performed using ultracentrifugation or commercial high-sensitivity RNA extraction kits; (3) CircRNA enrichment: strategies to enrich circRNAs for downstream analyses; (4) Detection methods: include qRT-PCR (sensitive, rapid), ddPCR (absolute quantification), and RNA-seq (global profiling); (5) Bioinformatic tools: circRNA analysis pipelines such as CIRCexplorer2, find_circ, and CIRI2, along with databases such as circBase, circRNADb, and CircInteractome; (6) Data analysis and interpretation: application of circRNA data in clinical translation and biomarker development [Created in BioRender. Singh DD (2025)]. circRNA: Circular RNA; EDTA: ethylenediaminetetraacetic acid; qRT-PCR: quantitative reverse transcription polymerase chain reaction; ddPCR: droplet digital polymerase chain reaction; RNA-seq: RNA sequencing.
The integration of these tools enables the development of dynamic circRNA-based biomarker panels that can provide real-time guidance for therapeutic decisions[95]. Although these approaches are still evolving, challenges remain in standardizing sample handling, accounting for pre-analytical variability, and differentiating tumor-derived circRNAs from those originating from non-malignant sources[16]. Nevertheless, the convergence of effective detection methods, sophisticated analytical methods, and key clinical investigations is positioning circRNA profiling as a key part of future precision oncology[96]. Looking ahead, liquid biopsies leveraging circRNAs to monitor therapeutic resistance are expected to increasingly support personalized, adaptive cancer treatment plans[2]. A comparison of RNA detection methods is presented in Table 5.
Comparison of RNA detection methods
| S.N. | RNA detection method | Technology/platform | Advantages | Disadvantages | Ref. |
| 1 | qRT-PCR | Fluorescence-based amplification (SYBR/Probe) | High sensitivity and specificity; cost-effective; fast turnaround; widely used for known targets | Requires prior knowledge of RNA; limited multiplexing; not ideal for novel discovery | [97] |
| 2 | ddPCR | Droplet partitioning + fluorescence detection | Absolute quantification; extremely sensitive; useful for low-abundance RNAs; high reproducibility | Higher cost; requires specialized equipment; limited throughput | [98] |
| 3 | Conventional RT-PCR | End-point PCR + gel electrophoresis | Simple and inexpensive; good for qualitative confirmation | Low sensitivity; not quantitative; risk of contamination | [99] |
| 4 | RNA-seq | NGS | Unbiased transcriptome-wide profiling; can detect novel RNAs including circRNAs; enables differential expression analysis | High cost; requires advanced bioinformatics; longer turnaround | [100] |
| 5 | Microarrays | Hybridization-based array platforms | Enable profiling of many RNAs at once; established technology | Limited to known sequences; lower dynamic range and sensitivity than RNA-seq | [101] |
| 6 | Northern blotting | RNA separation by gel + probe hybridization | Provides RNA size and abundance information; visual confirmation | Low sensitivity; labor-intensive; requires large RNA input | [102] |
| 7 | ISH | Tissue-based hybridization with labeled probes | Detects spatial localization of RNA in tissue or cells | Low throughput; semi-quantitative; needs tissue sections | [103] |
| 7 | NanoString nCounter | Barcoded probe-based digital counting | No need for amplification; highly multiplexed; works with degraded samples | Expensive; limited to pre-designed probes; not suitable for discovery | [104] |
CLINICAL TRIALS ON LIQUID BIOPSY-BASED DETECTION OF CIRCULATING CIRCRNAS FOR TRACKING DRUG RESISTANCE IN CANCER
Various preclinical and translational studies have demonstrated that circRNAs such as circFOXO3, circHIPK3, and F-circEA are involved in drug resistance pathways, including the evasion of apoptosis, regulation of autophagy, and drug efflux [Table 6][105]. Moreover, trials involving liquid biopsies have concentrated on ctDNA, CTCs, or miRNAs to monitor treatment response or early resistance in cancers such as breast, lung, and CRC[106]. For example, ctDNA-based clinical trials, including SERENA-6 by AstraZeneca and the NHS London pilot, have demonstrated the utility of early mutation detection in guiding therapy switches[107]. In contrast, circRNAs have not yet been integrated into routine clinical workflows[52]. Efforts have been made in observational studies and small cohort studies to study circRNAs in biofluids (especially in exosomes) as indicators of drug resistance in breast, prostate, and NSCLCs[111]. Importantly, these earliest studies provide proof-of-concept evidence that circRNA profiles can capture the dynamic evolution of a tumor’s adaptation to therapy[24]. Given the advances in sequencing and computational tools available for back-splice junction recognition, detecting circRNAs in clinical samples is technically feasible[112]. To translate these findings into clinical practice, dedicated trials are needed to evaluate the sensitivity, specificity, and predictive value of circRNA panels in monitoring drug resistance[113]. Future clinical investigations should focus on validating circRNA signatures in longitudinal patient samples, combining ctDNA and circRNA assays, and gradually integrating circRNAs into companion diagnostic workflows. Conducting such trials would represent an important milestone in personalized oncology, enabling real-time therapy adjustments based on evolving resistance patterns identified non-invasively[6]. Achieving this will require multi-center collaboration, standardized protocols for circRNA extraction and quantification, and regulatory support for biomarker-based decision making. As precision oncology advances, circRNA liquid biopsies represent a promising tool for early resistance detection and optimized therapy across multiple cancer types.
Summary of clinical and translational insights related to circRNA-based liquid biopsy for drug resistance monitoring
| S.N. | Trial/study name | Status | Target cancer type | Liquid biopsy marker | CircRNA focus | Detection method | Purpose/outcome | Ref. |
| 1 | F-circEA study | Completed (translational) | NSCLC | Plasma cfRNA/exosomes | F-circEA (from EML4-ALK) | qRT-PCR | Proof-of-concept: F-circEA detected in the plasma of ALK-positive patients; shows biomarker potential | [108] |
| 2 | CircRNA microarray study | Translational | Ovarian cancer | Plasma circRNAs | Multiple novel circRNAs | CircRNA microarray, RT-PCR | Identified differentially expressed circRNAs related to drug sensitivity | [109] |
| 3 | Pancreatic cancer circRNA panel study | Translational | PDAC | Plasma | 5-CircRNA diagnostic panel | RNA-seq + qRT-PCR | Proposed a diagnostic + drug response circRNA signature; preclinical stage | [95] |
| 4 | ctDNA-Guided SERENA-6 trial (AstraZeneca) | Phase III | Breast cancer | ctDNA | Not circRNA-specific | ddPCR/NGS | Demonstrated ctDNA-guided treatment switching improved PFS; circRNA potential underexplored | [107] |
| 4 | NHS London liquid biopsy pilot | Pilot program | Breast cancer | ctDNA | Not circRNA-specific | ddPCR | Applied ctDNA for ESR1 mutation detection; supports framework for circRNA integration | [110] |
LIMITATIONS OF THE STUDY ON CLINICAL TRIALS ON LIQUID BIOPSY-BASED DETECTION OF CIRCULATING CIRCRNAS FOR TRACKING DRUG RESISTANCE IN CANCER
Despite the growing interest in utilizing liquid biopsy for cancer management, clinical trials specifically focused on the detection of circulating circRNAs for monitoring drug resistance remain limited and face several important challenges[114]. One of the foremost limitations is the lack of ongoing or completed large-scale clinical trials that investigate the diagnostic or prognostic utility of circRNAs in a real-world therapeutic context[115]. Most available studies are preclinical or translational, primarily using in vitro cell lines or small patient cohorts, which limits their generalizability and clinical applicability[116]. The lack of robust clinical validation and longitudinal patient data makes it difficult to establish circRNAs as reliable biomarkers for tracking drug resistance. Additionally, the standardization of circRNA detection protocols presents a major barrier[117]. There is currently no universally accepted method for the enrichment, isolation, and quantification of circRNAs in clinical samples[118]. Techniques such as RNase R treatment, divergent primer design, and RNA seq are highly sensitive to experimental conditions, leading to variability in results across laboratories and studies[119]. Furthermore, the low abundance and tissue-specific expression of circRNAs often require advanced technologies such as ddPCR or deep RNA seq, which may not be available in routine diagnostic settings. Another major limitation lies in the functional characterization of identified circRNAs[52]. While many studies report associations between specific circRNAs and drug resistance phenotypes, few have established causal mechanisms or validated these findings in vivo[113]. This gap undermines the translational potential of circRNA signatures and limits their incorporation into clinical trial designs[120]. Moreover, circRNAs often function as competing endogenous RNAs or miRNA sponges, and their network-level interactions with other non-coding RNAs or mRNAs are complex and still not fully understood[84]. From a regulatory and ethical standpoint, the introduction of novel biomarkers into clinical trials necessitates rigorous validation under Good Clinical Practice and compliance with data safety and ethical standards, which adds time and logistical complexity to trial initiation and conduct[121]. Additionally, the cost and data burden of high-throughput circRNA profiling, especially when integrated with other omics data, may limit its widespread adoption, particularly in low-resource clinical settings[122].
Another issue is the lack of clear clinical utility or guidelines on how circRNA-based resistance detection would influence therapeutic decisions[123]. Unlike ctDNA, which can reveal actionable mutations leading to drug switching [e.g., estrogen receptor 1 (ESR1) mutations in breast cancer], circRNAs currently lack such validated clinical pathways[124]. Their integration into decision-making algorithms and treatment protocols remains theoretical at this stage. Lastly, patient heterogeneity in terms of tumor type, treatment history, and genetic background adds another layer of complexity, making it difficult to define universal circRNA panels for resistance tracking[125]. In conclusion, while the scientific foundation for using circulating circRNAs as non-invasive biomarkers for drug resistance is strong, the absence of clinical trial validation, methodological standardization, and real-time clinical utility assessment significantly limits current implementation. Addressing these limitations through collaborative, multi-center clinical trials and robust bioinformatics pipelines will be crucial for advancing the role of circRNAs in precision oncology.
FUTURE PROSPECTIVE
The significant potential of circRNAs in liquid biopsy for monitoring cancer drug resistance necessitates extensive future research, both experimental and clinical[44]. To begin, large-scale multi-center clinical trials should be launched to evaluate the clinical utility of circRNAs as predictive biomarkers of therapeutic resistance in multiple cancer types[112]. These trials should assess circRNA expression levels in solid tumors, hematological malignancies, and pediatric hematological malignancies using a longitudinal model[126]. The expression profiles will be correlated with resistance, progression-free survival (PFS), and therapeutic response to treatment modifications[120]. To ensure consistency and reproducibility, standardized pre-analytical and analytical methods are essential[44]. This includes establishing global standards for sample collection, circRNA extraction, RNase R-based enrichment, and detection using highly sensitive techniques (e.g., qRT-PCR, ddPCR, or RNA seq)[52]. Long-term data on circRNA expression will further enhance the clinical translatability of circRNAs as predictive biomarkers of therapeutic resistance, with far-reaching implications in cancer management.
The development of affordable, point-of-care diagnostic platforms for detecting tumor-specific circRNAs in body fluids could enable real-time monitoring of resistance while facilitating personalized treatment planning[127]. Future work should also prioritize functional characterization of resistance-associated circRNAs, including their molecular roles and interactions with miRNAs, mRNAs, and signaling pathways[85]. Such efforts would establish the biomarker potential of circRNAs and support the feasibility of therapeutically targeting oncogenic circRNAs. Combining circRNA profiles with other omics data (e.g., proteomics, genomics, metabolomics), using AI and machine learning approaches, may further enhance predictive accuracy and facilitate the development of comprehensive resistance signatures[128]. Another important step will be advancing circRNAs as companion diagnostics to guide precision-targeted therapies or immunotherapies[129]. Close collaboration with regulatory authorities will also be critical to define the biomarker qualification pathway for circRNAs while ensuring compliance with clinical and ethical standards[130]. In summary, advancing the field of circRNAs in liquid biopsy requires a multidisciplinary strategy that integrates molecular biology, bioinformatics, clinical oncology, and regulatory science. With sustained research investment and a strong translational focus, circRNAs hold the promise to transform non-invasive cancer monitoring and the precision management of therapeutic resistance.
CONCLUSION
Combining liquid biopsy with circRNA research is a revolutionary way to monitor drug resistance in cancer. circRNAs have a covalently closed-loop structure, making them highly stable in circulation and ideal candidates for non-invasive biomarkers in real-time disease monitoring. Advances in molecular testing such as qRT-PCR, ddPCR, and RNA seq have provided adequate sensitivity and specificity to detect circRNA from plasma, serum, and exosomes. In this way, circRNA can be linked to specific resistance mechanisms that include the regulation of apoptosis, autophagy, epithelial–mesenchymal transition, and drug efflux. By assessing therapeutic responses, circRNA-based liquid biopsy can capture molecular changes, enabling real-time interpretation of these dynamic events. Beyond this analytical purpose, liquid biopsy with circRNAs can guide treatment decisions, enable early detection of resistance, and improve personalized cancer care. Further standardization and clinical validation of such approaches are needed; nonetheless, current evidence supports the potential of circulating circRNAs as future clinical tools in precision oncology.
DECLARATIONS
Acknowledgments
The authors thank BioRender.com (July 17, 2025) for assistance with graphics. Singh DD acknowledges support from DST-FIST-AIMT and DST-PURSE at Amity University, Rajasthan, India. The graphical abstract was created with BioRender.com [Created in BioRender. Singh DD (2025)].
Authors’ contributions
Conceptualization, investigation, writing - original draft, review and editing: Singh DD
Conceptualization, investigation, writing - original draft, review and editing: Yadav DK
Supervision, resources, review and editing: Shin D
All authors have read and approved the final manuscript.
Availability of data and materials
Not applicable.
Financial support and sponsorship
This study was funded by the National Research Foundation of Korea (grant numbers: RS-2020-NR049589 and RS-2025-00555975). This work was also supported by the Gachon University Research Fund of 2022 (GCU-202206050001).
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Wang X, Zhang H, Chen X. Drug resistance and combating drug resistance in cancer. Cancer Drug Resist. 2019;2:141-60.
2. Ma L, Guo H, Zhao Y, et al. Liquid biopsy in cancer current: status, challenges and future prospects. Signal Transduct Target Ther. 2024;9:336.
3. Huang L, Rong Y, Tang X, Yi K, Wu J, Wang F. Circular RNAs are promising biomarkers in liquid biopsy for the diagnosis of non-small cell lung cancer. Front Mol Biosci. 2021;8:625722.
4. Batool SM, Yekula A, Khanna P, et al. The liquid biopsy consortium: challenges and opportunities for early cancer detection and monitoring. Cell Rep Med. 2023;4:101198.
5. Wen G, Zhou T, Gu W. The potential of using blood circular RNA as liquid biopsy biomarker for human diseases. Protein Cell. 2021;12:911-46.
6. Zhang Y, Wang Y, Su X, Wang P, Lin W. The value of circulating circular RNA in cancer diagnosis, monitoring, prognosis, and guiding treatment. Front Oncol. 2021;11:736546.
7. Wang S, Zhang K, Tan S, et al. Circular RNAs in body fluids as cancer biomarkers: the new frontier of liquid biopsies. Mol Cancer. 2021;20:13.
8. Babin L, Andraos E, Fuchs S, Pyronnet S, Brunet E, Meggetto F. From circRNAs to fusion circRNAs in hematological malignancies. JCI Insight. 2021;6:e151513.
9. Hua J, Wang Z, Cheng X, Dai J, Zhao P. Circular RNAs modulate cancer drug resistance: advances and challenges. Cancer Drug Resist. 2025;8:17.
10. Wu X, Shi M, Lian Y, Zhang H. Exosomal circRNAs as promising liquid biopsy biomarkers for glioma. Front Immunol. 2023;14:1039084.
11. Garlapati P, Ling J, Chiao PJ, Fu J. Circular RNAs regulate cancer-related signaling pathways and serve as potential diagnostic biomarkers for human cancers. Cancer Cell Int. 2021;21:317.
12. Choi SS, Kim SE, Oh SY, Ahn YH. Clinical implications of circulating circular RNAs in lung cancer. Biomedicines. 2022;10:871.
13. Li F, Yang Q, He AT, Yang BB. Circular RNAs in cancer: limitations in functional studies and diagnostic potential. Semin Cancer Biol. 2021;75:49-61.
14. Ghani MU, Du L, Moqbel AQ, et al. Exosomal ncRNAs in liquid biopsy: a new paradigm for early cancer diagnosis and monitoring. Front Oncol. 2025;15:1615433.
15. Liu XY, Zhang Q, Guo J, et al. The role of circular RNAs in the drug resistance of cancers. Front Oncol. 2021;11:790589.
16. Kundu I, Varshney S, Karnati S, Naidu S. The multifaceted roles of circular RNAs in cancer hallmarks: from mechanisms to clinical implications. Mol Ther Nucleic Acids. 2024;35:102286.
17. Zhang Q, Wang W, Zhou Q, et al. Roles of circRNAs in the tumour microenvironment. Mol Cancer. 2020;19:14.
18. Liu W, Niu J, Huo Y, et al. Role of circular RNAs in cancer therapy resistance. Mol Cancer. 2025;24:55.
19. Rochow H, Franz A, Jung M, et al. Instability of circular RNAs in clinical tissue samples impairs their reliable expression analysis using RT-qPCR: from the myth of their advantage as biomarkers to reality. Theranostics. 2020;10:9268-79.
20. Radanova M, Mihaylova G, Tasinov O, et al. New circulating circular RNAs with diagnostic and prognostic potential in advanced colorectal cancer. Int J Mol Sci. 2021;22:13283.
21. Bersani F, Picca F, Morena D, et al. Exploring circular MET RNA as a potential biomarker in tumors exhibiting high MET activity. J Exp Clin Cancer Res. 2023;42:120.
22. Roy S, Kanda M, Nomura S, et al. Diagnostic efficacy of circular RNAs as noninvasive, liquid biopsy biomarkers for early detection of gastric cancer. Mol Cancer. 2022;21:42.
23. Lu S, Liang Y, Li L, et al. Inferring circRNA-drug sensitivity associations via dual hierarchical attention networks and multiple kernel fusion. BMC Genomics. 2023;24:796.
24. Pisignano G, Michael DC, Visal TH, Pirlog R, Ladomery M, Calin GA. Going circular: history, present, and future of circRNAs in cancer. Oncogene. 2023;42:2783-800.
25. Wilusz JE. Circular RNAs: unexpected outputs of many protein-coding genes. RNA Biol. 2017;14:1007-17.
26. Liu X, Zhang Y, Zhou S, Dain L, Mei L, Zhu G. Circular RNA: an emerging frontier in RNA therapeutic targets, RNA therapeutics, and mRNA vaccines. J Control Release. 2022;348:84-94.
27. Nielsen AF, Bindereif A, Bozzoni I, et al. Best practice standards for circular RNA research. Nat Methods. 2022;19:1208-20.
28. Li Q, Wang Y, Wu S, et al. CircACC1 regulates assembly and activation of AMPK complex under metabolic stress. Cell Metab. 2019;30:157-73.e7.
29. Hussen BM, Abdullah SR, Jaafar RM, et al. Circular RNAs as key regulators in cancer hallmarks: new progress and therapeutic opportunities. Crit Rev Oncol Hematol. 2025;207:104612.
30. Yang Q, Li F, He AT, Yang BB. Circular RNAs: expression, localization, and therapeutic potentials. Mol Ther. 2021;29:1683-702.
31. Zhou M, Xiao MS, Li Z, Huang C. New progresses of circular RNA biology: from nuclear export to degradation. RNA Biol. 2021;18:1365-73.
32. Hashemi M, Khosroshahi EM, Daneii P, et al. Emerging roles of CircRNA-miRNA networks in cancer development and therapeutic response. Noncoding RNA Res. 2025;10:98-115.
33. Wei Z, Shi Y, Xue C, et al. Understanding the dual roles of circHIPK3 in tumorigenesis and tumor progression. J Cancer. 2022;13:3674-86.
34. Singh DD, Yadav DK, Shin D. Non-coding RNAs in cancer therapy-induced cardiotoxicity: unlocking precision biomarkers for early detection. Cell Signal. 2025;135:111982.
35. Singh DD, Kim Y, Choi SA, Han I, Yadav DK. Clinical significance of microRNAs, long non-coding RNAs, and circRNAs in cardiovascular diseases. Cells. 2023;12:1629.
36. Liu YY, Zhang LY, Du WZ. Circular RNA circ-PVT1 contributes to paclitaxel resistance of gastric cancer cells through the regulation of ZEB1 expression by sponging miR-124-3p. Biosci Rep. 2019;39:BSR20193045.
37. Xue C, Li G, Lu J, Li L. Crosstalk between circRNAs and the PI3K/AKT signaling pathway in cancer progression. Signal Transduct Target Ther. 2021;6:400.
38. Zhao M, Lin M, Zhang Z, Ye L. Research progress of circular RNA FOXO3 in diseases (review). Glob Med Genet. 2025;12:100003.
39. Shao Y, Song Y, Xu S, Li S, Zhou H. Expression profile of circular RNAs in oral squamous cell carcinoma. Front Oncol. 2020;10:533616.
40. Yan T, Tian X, Liu F, et al. The emerging role of circular RNAs in drug resistance of non-small cell lung cancer. Front Oncol. 2022;12:1003230.
41. Chen J, Yang J, Fei X, Wang X, Wang K. CircRNA ciRS-7: a novel oncogene in multiple cancers. Int J Biol Sci. 2021;17:379-89.
42. Han D, Li J, Wang H, et al. Circular RNA circMTO1 acts as the sponge of microRNA-9 to suppress hepatocellular carcinoma progression. Hepatology. 2017;66:1151-64.
43. Xia X, Li X, Li F, et al. A novel tumor suppressor protein encoded by circular AKT3 RNA inhibits glioblastoma tumorigenicity by competing with active phosphoinositide-dependent Kinase-1. Mol Cancer. 2019;18:131.
44. Xu T, Wang M, Jiang L, et al. CircRNAs in anticancer drug resistance: recent advances and future potential. Mol Cancer. 2020;19:127.
45. Zhang J, Luo Z, Zheng Y, Duan M, Qiu Z, Huang C. CircRNA as an Achilles heel of cancer: characterization, biomarker and therapeutic modalities. J Transl Med. 2024;22:752.
46. Adhit KK, Wanjari A, Menon S, Siddhaarth K. Liquid biopsy: an evolving paradigm for non-invasive disease diagnosis and monitoring in medicine. Cureus. 2023;15:e50176.
47. Wang M, Yu F, Li P, Wang K. Emerging function and clinical significance of exosomal circRNAs in cancer. Mol Ther Nucleic Acids. 2020;21:367-83.
48. Martinez-Dominguez MV, Zottel A, Šamec N, et al. Current technologies for RNA-directed liquid diagnostics. Cancers. 2021;13:5060.
49. Wang K, Bai X, Xue Y, et al. Absolute quantification of circRNA using digital reverse transcription-hyperbranched rolling circle amplification. Sens Actuators B Chem. 2023;375:132893.
50. Masante L, Susin G, Baudet M. Droplet digital PCR for the detection and quantification of bona fide CircRNAs. In: Dieterich C, Baudet M, editors. Circular RNAs. New York: Springer US; 2024. pp. 107-26.
51. Bauer-Negrini G, Cordenonsi da Fonseca G, Gottfried C, Herbert J. Usability evaluation of circRNA identification tools: development of a heuristic-based framework and analysis. Comput Biol Med. 2022;147:105785.
52. Gaffo E, Buratin A, Dal Molin A, Bortoluzzi S. Sensitive, reliable and robust circRNA detection from RNA-seq with CirComPara2. Brief Bioinform. 2022;23:bbab418.
53. Chen L, Wang C, Sun H, et al. The bioinformatics toolbox for circRNA discovery and analysis. Brief Bioinform. 2021;22:1706-28.
54. Zhang N, Wang X, Li Y, et al. Mechanisms and therapeutic implications of gene expression regulation by circRNA-protein interactions in cancer. Commun Biol. 2025;8:77.
55. Velpula T, Buddolla V. Enhancing detection and monitoring of circulating tumor cells: integrative approaches in liquid biopsy advances. J Liq Biopsy. 2025;8:100297.
56. Ren F, Fei Q, Qiu K, Zhang Y, Zhang H, Sun L. Liquid biopsy techniques and lung cancer: diagnosis, monitoring and evaluation. J Exp Clin Cancer Res. 2024;43:96.
57. Gao Y, Li C, Ji T, Yu K, Gao X. The biological function and mechanism of action of circRNA as a potential target in colorectal cancer. Crit Rev Oncol Hematol. 2025;213:104828.
58. Zhang Z, Yang T, Xiao J. Circular RNAs: promising biomarkers for human diseases. EBioMedicine. 2018;34:267-74.
59. Connal S, Cameron JM, Sala A, et al. Liquid biopsies: the future of cancer early detection. J Transl Med. 2023;21:118.
60. Feng XY, Zhu SX, Pu KJ, Huang HJ, Chen YQ, Wang WT. New insight into circRNAs: characterization, strategies, and biomedical applications. Exp Hematol Oncol. 2023;12:91.
61. Alimohammadi M, Kahkesh S, Khoshnazar SM, et al. Circular RNAs and doxorubicin resistance in cancer: molecular mechanisms and potential treatment targets. Gene. 2025;964:149636.
62. Siavashy S, Soltani M, Rahimi S, Hosseinali M, Guilandokht Z, Raahemifar K. Recent advancements in microfluidic-based biosensors for detection of genes and proteins: applications and techniques. Biosens Bioelectron X. 2024;19:100489.
63. Latifi-Pakdehi T, Khezrian A, Doosti-Irani A, Afshar S, Mahdavinezhad A. Investigating the biomarker value of circRNAs in the diagnosis of colorectal cancer: a systematic review. Discov Oncol. 2024;15:734.
64. Rashid S, Sun Y, Ali Khan Saddozai U, et al. Circulating tumor DNA and its role in detection, prognosis and therapeutics of hepatocellular carcinoma. Chin J Cancer Res. 2024;36:195-214.
65. Luo YH, Yang YP, Chien CS, et al. Circular RNA hsa_circ_0000190 facilitates the tumorigenesis and immune evasion by upregulating the expression of soluble PD-L1 in non-small-cell lung cancer. Int J Mol Sci. 2021;23:64.
66. Zeng K, He B, Yang BB, et al. The pro-metastasis effect of circANKS1B in breast cancer. Mol Cancer. 2018;17:160.
67. Wang X, Zhang H, Yang H, et al. Exosome-delivered circRNA promotes glycolysis to induce chemoresistance through the miR-122-PKM2 axis in colorectal cancer. Mol Oncol. 2020;14:539-55.
68. Huang X, Li Z, Zhang Q, et al. Circular RNA AKT3 upregulates PIK3R1 to enhance cisplatin resistance in gastric cancer via miR-198 suppression. Mol Cancer. 2019;18:71.
69. Dong ZR, Ke AW, Li T, et al. CircMEMO1 modulates the promoter methylation and expression of TCF21 to regulate hepatocellular carcinoma progression and sorafenib treatment sensitivity. Mol Cancer. 2021;20:75.
70. Salami R, Salami M, Mafi A, Vakili O, Asemi Z. Circular RNAs and glioblastoma multiforme: focus on molecular mechanisms. Cell Commun Signal. 2022;20:13.
71. Weidle UH, Birzele F. Deregulated circRNAs in epithelial ovarian cancer with activity in preclinical in vivo models: identification of targets and new modalities for therapeutic intervention. Cancer Genomics Proteomics. 2024;21:213-37.
72. Zhu Q, Zhang Y, Li M, et al. MiR-124-3p impedes the metastasis of non-small cell lung cancer via extracellular exosome transport and intracellular PI3K/AKT signaling. Biomark Res. 2023;11:1.
73. Xu A, Zhu L, Yao C, Zhou W, Guan Z. The therapeutic potential of circular RNA in triple-negative breast cancer. Cancer Drug Resist. 2024;7:13.
74. Li T, Wang WC, McAlister V, Zhou Q, Zheng X. Circular RNA in colorectal cancer. J Cell Mol Med. 2021;25:3667-79.
75. Cai X, Nie J, Chen L, Yu F. Circ_0000267 promotes gastric cancer progression via sponging MiR-503-5p and regulating HMGA2 expression. Mol Genet Genomic Med. 2020;8:e1093.
76. Han D, Li J, Wang H, et al. Circular RNA circMTO1 acts as the sponge of microRNA-9 to suppress hepatocellular carcinoma progression. Hepatology. 2017;66:1151-64.
77. Kong Z, Wan X, Lu Y, et al. Circular RNA circFOXO3 promotes prostate cancer progression through sponging miR-29a-3p. J Cell Mol Med. 2020;24:799-813.
78. Yang F, Liu DY, Guo JT, et al. Circular RNA circ-LDLRAD3 as a biomarker in diagnosis of pancreatic cancer. World J Gastroenterol. 2017;23:8345-54.
79. Morena D, Picca F, Taulli R. CircNT5E/miR-422a: a new circRNA-based ceRNA network in glioblastoma. Transl Cancer Res. 2019;8:S106-9.
80. Zeng XY, Yuan J, Wang C, et al. circCELSR1 facilitates ovarian cancer proliferation and metastasis by sponging miR-598 to activate BRD4 signals. Mol Med. 2020;26:70.
81. Su Y, Feng W, Shi J, Chen L, Huang J, Lin T. circRIP2 accelerates bladder cancer progression via miR-1305/Tgf-β2/smad3 pathway. Mol Cancer. 2020;19:23.
82. Zhu J, Li Q, Wu Z, Xu W, Jiang R. Circular RNA-mediated miRNA sponge & RNA binding protein in biological modulation of breast cancer. Noncoding RNA Res. 2024;9:262-76.
83. Kai D, Yannian L, Yitian C, Dinghao G, Xin Z, Wu J. Circular RNA HIPK3 promotes gallbladder cancer cell growth by sponging microRNA-124. Biochem Biophys Res Commun. 2018;503:863-9.
84. Huang Y, Zhang C, Xiong J, Ren H. Emerging important roles of circRNAs in human cancer and other diseases. Genes Dis. 2021;8:412-23.
85. He AT, Liu J, Li F, Yang BB. Targeting circular RNAs as a therapeutic approach: current strategies and challenges. Signal Transduct Target Ther. 2021;6:185.
86. Lone SN, Nisar S, Masoodi T, et al. Liquid biopsy: a step closer to transform diagnosis, prognosis and future of cancer treatments. Mol Cancer. 2022;21:79.
87. Hirahata T, Ul Quraish R, Quraish AU, Ul Quraish S, Naz M, Razzaq MA. Liquid biopsy: a distinctive approach to the diagnosis and prognosis of cancer. Cancer Inform. 2022;21:11769351221076062.
88. Verduci L, Strano S, Yarden Y, Blandino G. The circRNA-microRNA code: emerging implications for cancer diagnosis and treatment. Mol Oncol. 2019;13:669-80.
89. Wang H, Zhang Y, Zhang H, et al. Liquid biopsy for human cancer: cancer screening, monitoring, and treatment. MedComm. 2024;5:e564.
90. Lin D, Shen L, Luo M, et al. Circulating tumor cells: biology and clinical significance. Signal Transduct Target Ther. 2021;6:404.
91. Shi X, Wang B, Feng X, Xu Y, Lu K, Sun M. circRNAs and exosomes: a mysterious frontier for human cancer. Mol Ther Nucleic Acids. 2020;19:384-92.
92. Ge Q, Zhang ZY, Li SN, Ma JQ, Zhao Z. Liquid biopsy: comprehensive overview of circulating tumor DNA (Review). Oncol Lett. 2024;28:548.
93. Zhong P, Bai L, Hong M, et al. A comprehensive review on circulating cfRNA in plasma: implications for disease diagnosis and beyond. Diagnostics. 2024;14:1045.
94. Wang S, Dong Y, Gong A, et al. Exosomal circRNAs as novel cancer biomarkers: challenges and opportunities. Int J Biol Sci. 2021;17:562-73.
95. Xu C, Jun E, Okugawa Y, et al. A circulating panel of circRNA biomarkers for the noninvasive and early detection of pancreatic ductal adenocarcinoma. Gastroenterology. 2024;166:178-90.e16.
96. Nikanjam M, Kato S, Kurzrock R. Liquid biopsy: current technology and clinical applications. J Hematol Oncol. 2022;15:131.
97. Ma H, Bell KN, Loker RN. qPCR and qRT-PCR analysis: regulatory points to consider when conducting biodistribution and vector shedding studies. Mol Ther Methods Clin Dev. 2021;20:152-68.
98. Gerdes L, Iwobi A, Busch U, Pecoraro S. Optimization of digital droplet polymerase chain reaction for quantification of genetically modified organisms. Biomol Detect Quantif. 2016;7:9-20.
99. Hu T, Ke X, Yu Y, et al. NAPTUNE: nucleic acids and protein biomarkers testing via ultra-sensitive nucleases escalation. Nat Commun. 2025;16:1331.
100. Wang Z, Gerstein M, Snyder M. RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet. 2009;10:57-63.
101. Nazarov PV, Muller A, Kaoma T, et al. RNA sequencing and transcriptome arrays analyses show opposing results for alternative splicing in patient derived samples. BMC Genomics. 2017;18:443.
102. Yang T, Zhang M, Zhang N. Modified Northern blot protocol for easy detection of mRNAs in total RNA using radiolabeled probes. BMC Genomics. 2022;23:66.
103. Monné Rodríguez JM, Frisk AL, Kreutzer R, et al. European Society of Toxicologic Pathology (Pathology 2.0 Molecular Pathology Special Interest Group): review of in situ hybridization techniques for drug research and development. Toxicol Pathol. 2023;51:92-111.
104. Goytain A, Ng T. NanoString nCounter Technology: high-throughput RNA validation. In: Li H, Elfman J, editors. Chimeric RNA. New York: Springer US; 2020. pp. 125-39.
105. Wang S, Qian L, Cao T, et al. Advances in the study of circRNAs in tumor drug resistance. Front Oncol. 2022;12:868363.
106. Wang X, Wang L, Lin H, et al. Research progress of CTC, ctDNA, and EVs in cancer liquid biopsy. Front Oncol. 2024;14:1303335.
107. Turner N, Huang-Bartlett C, Kalinsky K, et al. Design of SERENA-6, a phase III switching trial of camizestrant in ESR1-mutant breast cancer during first-line treatment. Future Oncol. 2023;19:559-73.
108. Tan S, Gou Q, Pu W, et al. Circular RNA F-circEA produced from EML4-ALK fusion gene as a novel liquid biopsy biomarker for non-small cell lung cancer. Cell Res. 2018;28:693-5.
109. Ge L, Sun Y, Shi Y, et al. Plasma circRNA microarray profiling identifies novel circRNA biomarkers for the diagnosis of ovarian cancer. J Ovarian Res. 2022;15:58.
110. Borkar S, Markus F, Oetting A, et al. Detection of ESR1 mutations in tissue and liquid biopsy with novel next-generation sequencing and digital droplet PCR assays: insights from multi-center real life data of almost 6000 patients. Cancers. 2025;17:1266.
111. Kumar MA, Baba SK, Sadida HQ, et al. Extracellular vesicles as tools and targets in therapy for diseases. Signal Transduct Target Ther. 2024;9:27.
112. Hama Faraj GS, Hussen BM, Abdullah SR, et al. Advanced approaches of the use of circRNAs as a replacement for cancer therapy. Noncoding RNA Res. 2024;9:811-30.
113. Tao X, Ke X, Xu G. *Mechanisms of circular RNA in drug resistance of lung cancer: therapeutic targets, biomarkers, and future research directions. Discov Oncol. 2025;16:896.
114. Kirio K, Patop IL, Anduaga AM, et al. Circular RNAs exhibit exceptional stability in the aging brain and serve as reliable age and experience indicators. Cell Rep. 2025;44:115485.
115. Rashedi S, Mardani M, Rafati A, et al. Circular RNAs as prognostic and diagnostic biomarkers in renal cell carcinoma. J Clin Lab Anal. 2022;36:e24670.
116. Fosse V, Oldoni E, Bietrix F, et al; PERMIT group. Recommendations for robust and reproducible preclinical research in personalised medicine. BMC Med. 2023;21:14.
117. Kuwamoto-Imanishi S, Fujii H. Functions and potential clinical applications of circular RNAs in hepatocellular carcinoma. Hepatoma Res. 2025;11:15.
118. Shi H, Zhou Y, Jia E, et al. Comparative analysis of circular RNA enrichment methods. RNA Biol. 2022;19:55-67.
119. Karagianni K, Bibi A, Madé A, et al; EU-CardioRNA COST Action CA17129. Recommendations for detection, validation, and evaluation of RNA editing events in cardiovascular and neurological/neurodegenerative diseases. Mol Ther Nucleic Acids. 2024;35:102085.
120. Liu H, Hao W, Yang J, Zhang Y, Wang X, Zhang C. Emerging roles and potential clinical applications of translatable circular RNAs in cancer and other human diseases. Genes Dis. 2023;10:1994-2012.
121. Antoniou M, Kolamunnage-Dona R, Wason J, et al. Biomarker-guided trials: challenges in practice. Contemp Clin Trials Commun. 2019;16:100493.
122. Alqahtani S, Alqahtani T, Venkatesan K, et al. Unveiling pharmacogenomics insights into circular RNAs: toward precision medicine in cancer therapy. Biomolecules. 2025;15:535.
123. Malviya A, Bhuyan R. The recent advancements in circRNA research: from biogenesis to therapeutic interventions. Pathol Res Pract. 2023;248:154697.
124. Betz M, Massard V, Gilson P, et al. ESR1 gene mutations and liquid biopsy in ER-positive breast cancers: a small step forward, a giant leap for personalization of endocrine therapy? Cancers. 2023;15:5169.
125. Li Q, Geng S, Luo H, et al. Signaling pathways involved in colorectal cancer: pathogenesis and targeted therapy. Signal Transduct Target Ther. 2024;9:266.
126. Cui YB, Wang LJ, Xu JH, et al. Recent progress of circRNAs in hematological malignancies. Int J Med Sci. 2024;21:2544-61.
127. Li W, Liu JQ, Chen M, Xu J, Zhu D. Circular RNA in cancer development and immune regulation. J Cell Mol Med. 2022;26:1785-98.
128. de Gonzalo-Calvo D, Karaduzovic-Hadziabdic K, Dalgaard LT, et al. Machine learning for catalysing the integration of noncoding RNA in research and clinical practice. EBioMedicine. 2024;106:105247.
129. Pedraz-Valdunciel C, Rosell R. Defining the landscape of circRNAs in non-small cell lung cancer and their potential as liquid biopsy biomarkers: a complete review including current methods. Extracell Vesicles Circ Nucl Acids. 2021;2:179-201.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Topic
Copyright
Data & Comments
Data




















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.