Microvascular angiotensin II type 2 receptor function is enhanced in young females and declines in a model of murine aging
Abstract
Introduction: Angiotensin II (AngII) affects cardiovascular health, mediating impacts through AngII type 1 (AT1R) and type 2 (AT2R) receptors. The present study investigated sex and aging-related differences in microvascular AngII receptor function in mice and humans. Methods: Mesenteric resistance arteries (MRA) were isolated from
Keywords
INTRODUCTION
There are significant sex differences in the prevalence and rate of progression of cardiovascular diseases across the lifespan, including in myocardial infarction, stroke, heart failure, and hypertension[1,2], yet the mechanisms for these sexual dimorphisms remain largely unknown. The average age of hypertension onset and cardiovascular disease development is delayed in women compared with men, with prevalence increasing in women after menopause and ultimately exceeding that of men[1]. To date, sex-specific therapeutic targets for hypertension and cardiovascular disease have yet to be identified, which is a critical step toward improving cardiovascular outcomes and attenuating health disparities in women across the lifespan.
The renin-angiotensin-aldosterone system (RAAS) is a hormonal cascade that regulates blood pressure and plays an important role in cardiovascular health and disease[3-5]. Components of the RAAS are altered with aging in humans and rodents, including the levels of circulating hormones, expression of the enzymes that produce these hormones, and the receptors that mediate their impacts on cardiovascular tissues[3-6]. Angiotensin II (AngII) is a circulating peptide hormone and a main effector of the RAAS. There are two main receptor subtypes through which AngII exerts its effects: AngII type 1 receptors (AT1R) and type 2 receptors (AT2R)[7]. The AT1R exerts classical AngII effects in the cardiovascular system, including mediating potent vasoconstriction[7]. Conversely, the AT2R exerts opposing cardiovascular effects, specifically inducing vasodilation[7-11], which modulates the vasoconstrictive and pro-hypertensive actions of Ang II.
The microvasculature is an important contributor to blood pressure regulation by controlling peripheral vascular resistance. As such, the balance between AT1R- and AT2R-mediated effects in the microvasculature contributes to the development of hypertension[12] and other cardiovascular conditions[9]. Both AT1R and AT2R are expressed in endothelial cells and vascular smooth muscle cells in mesenteric resistance arteries (MRA) in rodents[9,10]. AT2R expression is greatest during development, and though it declines to lower absolute and relative (vs. AT1R) levels in adulthood[9,12-15], the AT2R functionally contributes to blood pressure control, renal physiology, and microvascular function in adult rodent models[10,12] and humans[15-17]. These findings, plus the recent development of AT2R agonists for human health[18,19], heighten interest in the role of AT2R in the cardiovascular system.
Data from animal models and recent human studies suggest sex- and aging-related differences in AT2R function and expression in the cardiovascular system[11,16,17,20-23]. The gene that encodes for AT2R is located on the X chromosome[24], and estrogen and testosterone modulate AT2R expression in the vasculature of rodents[25,26]. Sex hormone receptors also mediate AngII-induced hypertension and AngII downstream signaling[9,20-22]. In humans, young women have enhanced cutaneous microvascular vasodilation in response to AT2R activation[17], and older adults have enhanced microvascular constriction in response to AngII than younger adults, a difference which can be abolished by AT2R inhibition[16]. Detailed investigation of the contribution of AT2R to microvascular function during aging has been limited and many preclinical studies have been performed only in male rodents; thus, there is a knowledge gap regarding potential sex differences in microvascular AngII receptor function in young and aged models.
Here, we investigate sex differences in AngII-induced microvascular constriction and AT2R-mediated dilation in an aging murine model and relate the murine model findings with clinical microvascular function studies in humans. We hypothesized that: (1) AngII-induced constriction would increase later in life in female compared with male mice; (2) AT2R function would be greater in female compared with male mice and lost with aging; and (3) AngII vasoconstriction in young female mice and women would be reduced compared to males and that this difference would be abolished with AT2R antagonism.
MATERIALS & METHODS
Animal studies
All mice were handled in accordance with US National Institutes of Health standards, and all procedures were approved by the Tufts University Institutional Animal Care and Use Committee (Protocol #:
Mesenteric vessel wire myograph studies
Rings from second- and third-order mesenteric resistance arteries (MRA) were mounted in a myograph (Danish Myo Technologies) for isometric tension recordings using PowerLab software (AD Instruments). A total of four rings per mouse were used for each wire myograph study. Rings were placed under a resting tension of 2 milliNewtons (mN) in tissue baths containing warmed (37 °C), aerated (95% O2, 5% CO2) standard physiological saline solution (PSS) (in mM: 130 NaCl, 4.7 KCl, 1.17 MgSO4, 0.03 EDTA, 1.6 CaCl2, 14.9 NaHCO3, 1.18 KH2PO4 and 5.5 glucose). Administration of 10 μM phenylephrine (PE) was used to test arterial viability, and the presence of intact endothelium was verified by acetylcholine (Ach, 1 μM)-induced relaxation of a half-maximal PE-induced contraction. Only vessels that met these criteria (intact SMC and endothelium) were included in the results. For vasoconstriction studies, four concentrations of AngII were administered (1 × 10-9, 3 × 10-9, 1 × 10-8, 1 × 10-7 M) to four separate mesenteric vessel rings (one concentration per ring due to AT1R internalization), and raw force was measured in mN. For vasodilation studies, vessels were pre-treated with 10 μm losartan for 30 min to block AT1R. Vessels were then preconstricted with PE and a dose-response of AT2R agonist compound-21 (C21, a gift from Vicore Pharma;
Quantitative RT-PCR
Total RNA was extracted from mouse MRA, reverse transcribed, and quantitative RT-PCR was performed with gene-specific primers, as previously described[28,29]. Each N represents MRA from two mice pooled together to maximize the total RNA isolated. Ct values were normalized to β2-microglobulin (B2m). Primer sequences are specified in Supplementary Table 1.
Human microvascular function studies
In vivo studies in humans were approved by the Tufts Health Sciences Institutional Review Board and conformed to the guidelines set forth by the Declaration of Helsinki. All participants gave verbal and written informed consent before participation. Seven healthy individuals (3 premenopausal women and 4 age-matched men) were studied. Participants were excluded for: history of myocardial infarction, heart failure, cardiovascular diseases, diabetes, chronic diseases, and current tobacco use. Cutaneous microvascular function was measured as previously described[30,31]. Descriptive data for participants is presented in Supplementary Table 2.
Females were studied during the early follicular phase of the natural menstrual cycle or in the placebo pill phase of oral contraceptive pill use to minimize the influence of female sex hormones. Participants arrived at the laboratory after an overnight fast and abstained from alcohol/caffeine for 12 h and vigorous exercise for 24 h. Height, weight, and resting blood pressure were measured. Two microdialysis fibers (CMA 31; Harvard Apparatus, Holliston, MA) were inserted in the ventral side of the non-dominant forearm of each participant, as previously described[30-33]. A 25-gauge needle was inserted into the dermis after a 10-min application of ice to the skin surface to provide short-term local anesthesia. The MD fibers were then threaded through the lumen of the needle, which was removed once the semi-permeable section of the fiber was in place. The MD fibers were taped to the skin, and Ringer’s solution was perfused through all sites for at least 60 min or until the local hyperemia associated with fiber insertion subsided.
Skin blood flow was measured as cutaneous red blood cell (RBC) flux from 1.5 mm2 of skin with a multifiber laser Doppler probe placed in a local heater (MoorLAB, Temperature Monitor SH02, Moor Instruments, Devon, UK). Brachial blood pressure was measured every 10 min on the contralateral arm by an automated oscillometric sphygmomanometer (GE Healthcare, Dinamap ProCare 400 Vital Signs Monitor, Chicago, IL, USA). Following the hyperemia resolution period, one site was randomly assigned to receive the AT2R antagonist PD123319 (1 µM, Tocris) and the other site received Ringer’s solution as a control. Following 1 h of pre-treatment with PD123319 or Ringer’s solution, both sites received the same incremental concentrations of AngII from 10-9 to 10-4 M to elicit vasoconstriction[16].
RBC flux data were collected at 40 Hz using the PL3516 PowerLab data acquisition system and LabChart software (AD Instruments, Colorado Springs, CO). Cutaneous vascular conductance (CVC) was calculated as RBC flux divided by mean arterial pressure and standardized to site-specific baseline (%CVCbaseline).
Human cell culture studies and immunoblotting
De-identified human aortic tissue was obtained post-mortem from the NIH-supported National Disease Research Interchange, and hence, the medical ethics committee of participating center (Tufts Medical Center) deemed this research to be exempt from human subjects’ research requirements. Human aortic smooth muscle cells (HAoSMC) and endothelial cells (HAoEC) from premenopausal women and age-matched men were compared. For HAoEC isolation, aorta tissues were enzymatically digested by dispase (Gibco) to create single EC suspensions. Pure ECs were isolated using magnetic beads conjugate EC-specific antibody (Invitrogen). HAoECs were cultured in endothelial cell medium (Cell Biologics) and grown to ~90% confluency before collection. HAoSMC were isolated by the explant method, as previously described[18], and used only to passage 8.
The cell lysates in protein sample buffer were incubated for 5 min at 95 °C, run on a 10% SDS PAGE gel, transferred to PVDF Transfer Membrane (Millipore), blocked with 5% non-fat milk, and probed with appropriate primary antibodies (AT2R and AT1R, Abcam; GAPDH, Cell Signaling). Secondary antibodies used were anti-mouse and anti-rabbit horseradish peroxidase secondary antibody (Cell Signaling). Blots were imaged using ECL reagent (Fisher).
Statistical analysis
Group differences were assessed using one-way analysis of variance (ANOVA), two-way ANOVA, repeated-measures two-way ANOVA, or Student’s t-test as indicated in each figure legend. Tukey post hoc testing was used for all post hoc pairwise comparisons. Effect size (Cohen’s d) and 95% confidence interval (CI: lower limit, upper limit) are displayed as necessary for data transparency. The level of significance was set at α = 0.05 for all statistical tests. All data are presented as mean ± standard error of the mean (SEM).
RESULTS
Angiotensin II-induced vasoconstriction increases later in life in female compared with male mice
To examine sex-specific, aging-related changes in microvascular AngII-induced constriction, MRA were isolated from 3, 12, and 18-month-old female and male mice and constriction to AngII was determined. The response to AngII is a composite measure of constriction driven by the AT1R and dilation due to AT2R activity, with AT1R function dominating and resulting in an overall constrictive response. In female mice, AngII-induced constriction was greater in 18-month-old compared with both 3- and 12-month-old mice, with no difference in vasoconstriction at any concentration between 3- and 12-month-old mice
Figure 1. Angiotensin II-induced vasoconstriction increases later in life in female compared with male mice. Second- and third-order mesenteric resistance arteries were isolated from 3-, 12-, and 18-month-old C57/Bl6 mice. Four separate segments from each mouse were hung in a wire myograph and administered a different concentration of angiotensin II (1 × 10-9 to 1 × 10-7 M). Microvascular constriction was measured in vessels from (A) females and (B) males in response to angiotensin II concentrations by wire myography. Data are expressed as raw force in milliNewtons (mN) from baseline. N = 3-4/group (all female groups and 3-month-old males = 3/group; 12- and 18-month-old males = 4/group). The main effects of concentration and age and the interaction effect of these two factors were assessed via repeated measures two-way ANOVA with Tukey post hoc testing where appropriate. Data are means ± SEM,
AT2R-mediated vasodilation declines with age in female mice only
To determine if differences in AT2R activity contribute to the sex differences in microvascular function, MRA were pre-treated with losartan to block AT1R, preconstricted with PE, and then administered increasing concentrations of AT2R agonist C21. There were no statistical differences in the preconstriction response to PE between compared groups (young females: 3.1 mN ± 0.4, aged females: 3.2 mN ± 0.3, young males: 2.9 mN ± 0.2, aged males 3.2 mN ± 0.3). In female mice [Figure 2A], there was no difference in AT2R-mediated dilation at any concentration between 3 and 12-month-old mice. Compared with
Figure 2. AT2R-mediated vasodilation declines with age in female mice only. Second- and third-order mesenteric resistance arteries were isolated from 3-, 12-, and 18-month-old C57/Bl6 (A) female and (B) male mice. Vessels were pre-treated with losartan for 30 min to block angiotensin II type 1 receptors. Following preconstriction to phenylephrine (PE), vasorelaxation in response to Compound 21 (AT2R agonist, 1 × 10-12 to 1 × 10-7 M) was determined by wire myography. Data are expressed as percent relaxation from PE preconsriction. N = 3-7/group (18-month-old males and females = 3/group; 3-month-old females and males and 12-month-old males = 4/group; 12-month-old females = 7/group). The main effects of concentration and age and the interaction effect of these two factors were assessed via repeated measures two-way ANOVA with Tukey post hoc testing where appropriate. Data are means ± SEM,
Mesenteric resistance artery AT2R mRNA declines with age in female mice only
Next, we examined whether the age-dependent changes in AT2R activity may be mediated by changes in MRA AT2R expression. AngII receptor mRNA expression was quantified in mouse MRA [Figure 3]. In female mice, AT2R mRNA decreased in 12- and 18-month-old mice vs. 3-month-old mice [Figure 3A]. There was no difference in AT2R expression with age in MRA from male mice [Figure 3B]. We observed no difference in AT1RB mRNA expression with aging in female [Figure 3C] or male [Figure 3D] mice.
Figure 3. Mesenteric resistance artery AT2R mRNA declines with age in female mice only. Total RNA was extracted from mesenteric resistance arteries, and qRT/PCR was performed to quantify angiotensin II type 2 [AT2R, (A and B)] and type 1b [AT1BR,
Angiotensin II-induced vasoconstriction is attenuated in young female compared with male mice, and this difference is abolished with AT2R inhibition
To determine if AT2R activity mediates sex differences in AngII-induced constriction, wire myography studies were performed to quantify AngII-induced constriction (AngII dose
Figure 4. Angiotensin II-induced vasoconstriction is attenuated in young female compared with male mice, and this difference is abolished with AT2R inhibition. Second- and third-order mesenteric resistance arteries were isolated from 3-month-old C57/Bl6 female and male mice. Vessels were pre-treated with vehicle (circles) or 10 μM PD123319 (squares), an AT2R antagonist. Vessel constriction in response to AngII (1 × 10-7 M) was determined by wire myography. Data are expressed as raw force in milliNewtons (mN). N = 8-10/group. The main effects of treatment and sex and the interaction effect of these two factors were assessed via two-way ANOVA with Tukey post hoc testing where appropriate. Data are means ± SEM, *P < 0.05.
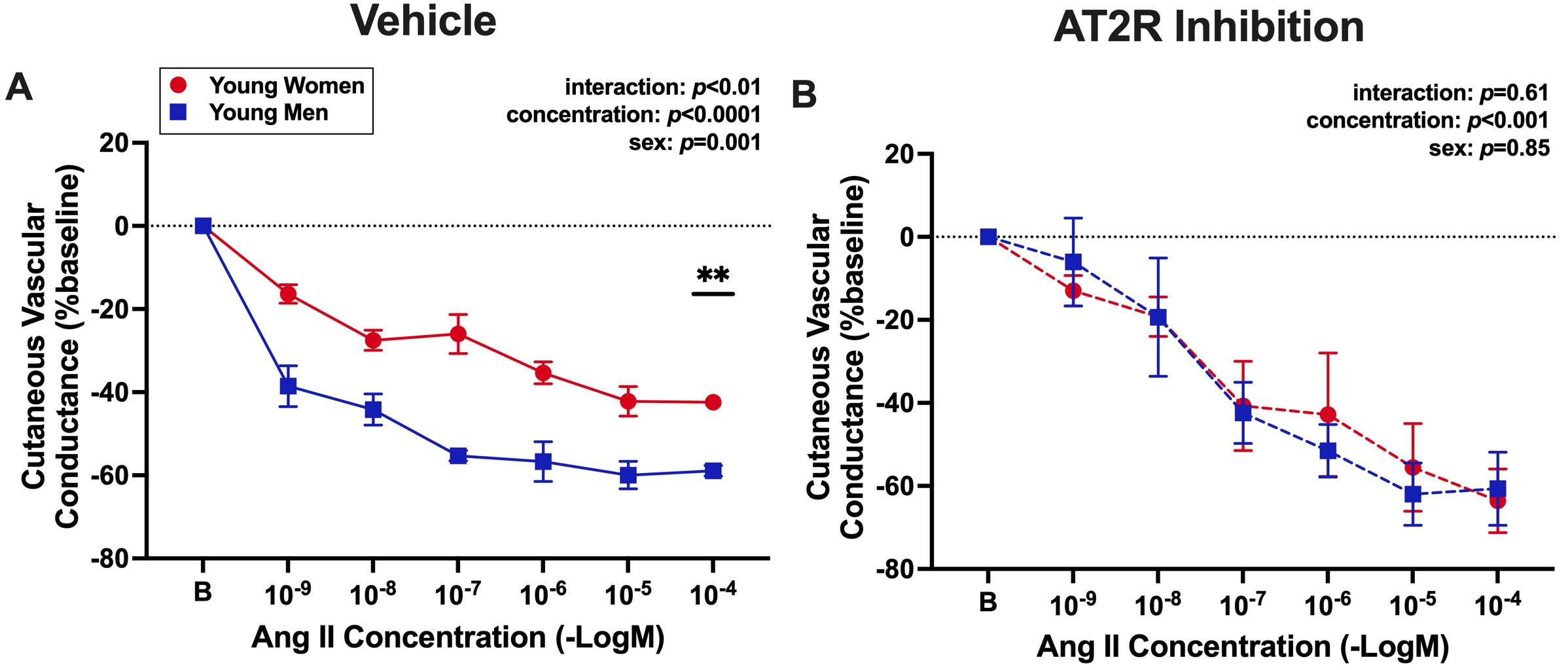
Angiotensin II-induced vasoconstriction is attenuated in premenopausal women compared with men, and this difference is abolished with AT2R inhibition
To translate murine findings to humans, cutaneous microvascular constriction to AngII in the presence and absence of AT2R antagonist, PD123319, was assessed in healthy women and men. For the presentation of data in Figure 5, the decline in cutaneous vascular conductance in response to AngII corresponds to the degree of vasoconstriction of the cutaneous microvasculature. There was a significant effect of sex at vehicle sites, where women constricted less to AngII compared with men [Figure 5A]. There was also a significant interaction effect of sex and concentration at vehicle sites [Figure 5A], such that vasoconstriction at
Figure 5. Angiotensin II-induced vasoconstriction is attenuated in premenopausal women compared with men, and this difference is abolished with AT2R inhibition. Forearm cutaneous vascular conductance was assessed to measure microvascular constriction in response to increasing concentrations of AngII (1 × 10-9 to 1 × 10-4 M) in young, premenopausal women (N = 3, red circles, average age: 28 ± 2 years) and young, age-matched men (N = 4, blue squares, average age: 30 ± 3 years). Local skin sites were pre-treated with (A) lactated Ringer’s (vehicle control) or (B) an AT2R antagonist (1 μM PD123319). Cutaneous vascular conductance (red blood cell flux/mean arterial pressure) is expressed as a percentage of baseline. The decline in cutaneous vascular conductance in response to AngII corresponds to the degree of vasoconstriction of the cutaneous microvasculature. The main effects of concentration and sex and the interaction effect of these two factors were assessed via repeated measures two-way ANOVA with Tukey post hoc testing where appropriate. Data are means ± SEM, **P < 0.01.
AT2R expression in human aortic smooth muscle cells is greater in premenopausal women vs. men
To elucidate whether there are sex differences in vascular cell expression of AngII receptors, protein expression of AT2R and AT1R was determined in primary HAoSMC and HAoEC from young women
Figure 6. AT2R expression in human aortic smooth muscle cells is greater in premenopausal women vs. men. The expression of angiotensin II type 2 [AT2R,
DISCUSSION
The novel findings from the present study are that: (1) in female mice, there is a concurrent loss of microvascular AT2R-mediated dilation and an increase in AngII-induced constriction with advancing age; (2) microvascular AngII-induced constriction increases earlier in males with no aging-associated change in AT2R-mediated dilation; (3) the decline of AT2R-mediated dilation with aging in female mice corresponds with a decrease in microvascular AT2R mRNA expression; (4) young female mice have attenuated microvascular AngII-induced constriction compared with young male mice, and this sex difference is driven by enhanced AT2R function; and (5) clinical data from premenopausal women and age-matched men support the sex difference in microvascular AT2R function seen in mice, such that young women have attenuated AngII-induced constriction compared with men, which is abolished with AT2R inhibition, indicating enhanced AT2R function in young women. This is associated with greater AT2R protein expression in HAoSMC from premenopausal women. Overall, these findings demonstrate that young females have enhanced microvascular AT2R expression and function, and that female microvascular aging is driven by both a loss of AT2R-mediated dilation and an increase in AngII-induced vasoconstriction, while male microvascular aging is primarily driven by an increase in AngII-induced vasoconstriction with no alterations in AT2R function.
The prevalence of cardiovascular disease increases with aging in both men and women, but this occurs in a sexually dimorphic pattern[1]. In general, premenopausal women are protected from cardiovascular disease and develop CVD later in life compared with men[1]. However, the mechanisms that mediate the sex-specific time course of CVD progression are not well understood. There are validated sex differences in the RAAS and known changes in levels of RAAS components (including AngII and its receptors) with aging[4,34]. However, there has been limited investigation of sex differences in vascular RAAS alterations with aging, specifically in the microcirculation. Prior studies have suggested that sex differences in responses to AngII may be mediated by differences in AT1R expression and activity or AngII synthesis, which can be regulated by sex hormone exposure[21,35]. Findings from the current study identify differences in AT2R function and expression as a sex-specific mechanism of microvascular aging.
Previous work shows that the AT2R contributes to vasodilation under healthy conditions[7-11]. There is evidence in various vascular beds that AT2R stimulation yields vasodilation via bradykinin, nitric oxide (NO), cyclic guanosine monophosphate, and cytochrome P-450-dependent (but NO-independent) pathways[36]. More specifically, C21-mediated activation of AT2R induces NO release through PKA/phosphorylated endothelial-derived NO synthase (p-eNOS) and AKT/p-eNOS signaling pathways[37]. Previous studies have identified a vasodilatory role of AT2R in the microvasculature of normotensive male rats, but this is reversed to a vasoconstrictive role in the setting of aging[38] and hypertension[10,12]. Importantly, AT2R function is restored to a vasodilatory response when blood pressure is normalized in the aging and hypertensive models[10,12,38]. The current findings are consistent with previous reports, as our results show that males exhibit only modest AT2R-mediated dilation at any age. One difference is that our study directly measured AT2R-mediated dilation with C21, whereas the previous studies assessed the vascular response to AngII in the presence and absence of AT2R inhibition, which may contribute to slightly differing results in young males between the current study and previous findings. The alterations we observed in AT2R function in females coincided with a reduction in microvascular AT2R mRNA expression in females but not males. The present study also provides evidence that AT2R represses microvascular AngII-induced constriction in young female but not male mice. These findings support that microvascular AT2R function is enhanced and protective in young females but not males. Clinical data demonstrate that women are more likely to have non-obstructive coronary artery disease attributable to coronary microvascular dysfunction[39], for which there are no current sex-specific treatment options. The current findings suggest that restoration of AT2R expression and function may be an effective sex-specific treatment in the management and prevention of aging-associated cardiovascular disease in women. It is also plausible that AT2R agonist therapy may be beneficial in males, but future studies are required to determine this.
There is strong evidence that sex differences in AT2R function contribute to blood pressure responses in the setting of RAAS activation. Multiple studies have shown that young female rodents have a reduced pressor response to AngII compared with males[11,20-22]. This has also been shown in response to acute AngII infusion in young, healthy women compared with men[40]. Young female mice exhibit a decrease in mean arterial pressure in response to low-dose AngII, which can be reversed by AT2R antagonism, while males have no response[11,41]. This AT2R-mediated reduction in arterial pressure is estrogen-dependent in females[42]. Similarly, aged, reproductively senescent females also demonstrate an AT2R-mediated reduction in AngII-induced increases in blood pressure when supplemented with estrogen[43]. Cerebral AT2R is also protective against the development of mineralocorticoid receptor- plus salt-induced hypertension in female, but not male, rats[44]. In the renal system, AT2R in female rodents maintains autoregulation of renal blood flow and glomerular filtration rate at low renal perfusion pressures[45], counteracts renal pressor responses to AngII[45], and mediates the pressure-natriuresis relationship, such that young females excrete the same amount of sodium as young males but at a lower arterial pressure[46]. Interestingly, the AT2R-dependent effect on the pressure-natriuresis relationship is lost with aging in female rodents, which coincides with a reduction in the ratio of AT2R to AT1R mRNA in the kidney with aging[46]. Findings from the present study further our understanding of how sex differences in AT2R function may contribute to hypertension and cardiovascular disease, adding that the loss of enhanced microvascular AT2R activity in females may contribute to the aging-associated increase in hypertension and cardiovascular diseases in aging women.
Obesity and pre-diabetes are also risk factors for cardiovascular disease and increase with aging. Obesity is also more common in women[1], and when associated with pre-diabetes, obesity mitigates the protection from CVD seen in premenopausal women[47]. The development of obesity with pre-diabetes in rats reduces cardiac AT2R expression in females but not males[48]. Further, increasing AT2R expression in the heart can mitigate cardiac damage induced by obesity and pre-diabetes in male and female rats[49,50]. Future studies are warranted to determine whether exposure to obesity accelerates the aging-related decline in microvascular AT2R function as a mechanism for promoting cardiovascular disease risk in women.
There are substantial available data demonstrating a role for sex hormones and sex hormone receptors in regulating AT2R expression. Overall, expression of AT2R is greater in females compared with males in many tissues, including the kidney, heart, vasculature, adrenal glands, and the central and peripheral nervous systems[9,11,48]. Several mechanisms have been implicated in determining this sex difference[9]. First, the gene encoding for AT2R resides on the X chromosome[24]; hence, the dosage effect of the two X chromosomes may contribute to greater expression in females. There are also estrogen-responsive elements in the promoter region of the AT2R gene, and estrogen upregulates the expression of AT2R in many tissues[9,26,51,52]. Conversely, testosterone downregulates AT2R expression in the aorta[25], which may contribute to lower expression of AT2R in males. Upregulation of AT2R expression by estrogen is dependent on estrogen receptor β in uterine arteries from pregnant rats[26] and downregulation of AT2R by testosterone is androgen receptor-dependent in the aorta of female rats[25]. Protection from AngII-induced increases in blood pressure in female rodents is also estrogen receptor α-dependent[21]. The average age of cessation of normal estrous cycles in female C57/Bl6 mice occurs between 11 and 15 months of age[27]. Therefore, a reduction in circulating estrogens may contribute to the results in the present study, though this cannot be confirmed with the present data, as we did not measure circulating estrogens in our female mice. Future studies on the role of sex hormones and sex hormone receptors on AT2R expression and function in the microvasculature during aging are warranted.
Our clinical study adds to the growing literature supporting the role of AT2R in microvascular function in women. As we show in young female mice, premenopausal women have attenuated microvascular constriction to AngII compared with age-matched men, and this difference is abolished with AT2R inhibition. Other clinical studies have recently demonstrated both sex- and aging-related differences in AT2R function in human cutaneous microvascular responses. Lang and Krajek[16] found that AT2R activity attenuates microvascular AngII-induced constriction in mixed-sex cohorts of young adults but not older adults. More recently, Schwartz et al. found that AT2R-mediated dilation in response to C21 is greater in young women compared with young men[17]. An earlier study by Stewart et al. showed that AngII increased microvascular vasodilation during concurrent AT1R inhibition and NO synthase inhibition, but that additional inhibition of AT2R (with PD123319) had no effect[53]. This study was completed in a young, mixed-sex cohort (5 men, 3 women)[53], and hence, it remains unclear how sex differences may have affected this outcome. Overall, our results identify a novel mechanism driving the sex difference in the cutaneous microvascular response to AngII, with attenuated vasoconstriction in premenopausal women that is attributable to enhanced AT2R activity.
Our data also show greater AT2R protein expression in HAoSMC from premenopausal women compared with men, but no differences in AT2R expression in HAoEC or AT1R expression in either cell type. This suggests a greater AT2R to AT1R ratio in smooth muscle cells from premenopausal women compared with men, which may contribute to sex differences in AngII effects on vascular function outcomes in humans. These data also suggest that AT2R in SMC, but not EC, may be a potential driver of cardiovascular protection in young females. However, previous literature also suggests that AT2R may have varying effects by vascular bed, as estrogen supplementation in aged, reproductively-senescent female mice showed AT2R-dependent attenuation in AngII-induced increases in blood pressure but had no effect on aortic vasodilator function[43]. It is important to note that the present studies assessing human protein expression of AngII receptors were completed with aortic vascular cells, and further studies are needed to confirm these findings in human microvascular SMC and EC, which are less readily available.
Limitations
This study has several limitations that should be considered when interpreting the results. First, we did not measure blood pressure in the murine aging model; however, previous studies have shown that young male mice have modestly higher basal blood pressure than young female mice[54] and that male mice develop a modest increase in blood pressure by 7 months of age[55]. Potential aging-associated alterations in blood pressure in female mice are still unknown and warrant future studies. Third, we did not measure the protein expression of AngII receptors in mice. This is largely due to the lack of reliable antibodies for AT1R in mice. In addition, our study design did not address how endothelial function may influence AT2R-mediated vasodilation. Future studies are required to dissect the role of endothelial function in age and sex differences in AT2R-mediated vasodilation. Next, due to limited recruitment capacity, we were not able to measure human microvascular function in older adults; thus, further investigation of sex differences in microvascular AngII receptor activity in human aging is necessary. Additionally, we measured AngII receptor expression in human aortic cells, which may differ from what occurs at the microvascular level in humans. Nonetheless, we did detect sex differences in aortic SMC AT2R expression, suggesting that aortic AT2R may also be relevant in female vascular health. Lastly, we recognize that we have low sample sizes in some of our mouse mesenteric function data. Although our data are significant, future studies should confirm with larger sample sizes.
Perspectives
Overall, the present study identifies enhanced microvascular AT2R activity in young female compared with male mice and demonstrates a decline in microvascular AT2R expression and AT2R-mediated vasodilation as a novel mechanism of female vascular aging. The clinical data provide novel translational evidence of sex differences in AT2R function in the human microvasculature. These results nominate the AT2R as a potential sex-specific therapeutic target for microvascular dysfunction and the consequential development of cardiovascular disease in aging women. As AT2R agonists have already been successfully used in clinical trials in humans, these findings have the potential to be rapidly translated. Future investigation is essential to determine the precise molecular mechanisms regulating microvascular AT2R function and how this differs by sex and age to contribute to the differential manifestation of aging-associated cardiovascular diseases in women and men.
DECLARATIONS
Acknowledgments
The authors would like to thank Rachel Kenney and Jennifer Vorn for their assistance with data collection.
Authors’ contributions
Contributed to the conception of the study and drafted the manuscript: DuPont JJ, Turner CG
Performed experiments, analyzed data, and interpreted results: Turner CG, de Oliveira K, Lu Q, Patel AR, Pulakat L, Jaffe IZ, DuPont JJ
Critically revised and approved the final version of the submitted manuscript: Turner CG, de Oliveira K,
Availability of data and materials
Not applicable.
Financial support and sponsorship
This work is supported by the National Institutes of Health (R01HL160834 and 1K12 HD092535-01 to DuPont JJ) and the American Heart Association (https://doi.org/10.58275/AHA.24POST1192551.pc.gr.190835 to Turner CG).
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
All mice were handled in accordance with US National Institutes of Health standards, and all procedures were approved by the Tufts University Institutional Animal Care and Use Committee (Protocol #: B2023-83).
Consent for publication.
Not applicable.
Copyright
© The Author(s) 2024.
Supplementary Materials
REFERENCES
1. Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics-2023 update: a report from the american heart association. Circulation 2023;147:e93-621.
2. DeFilippis EM, Van Spall HGC. Is it time for sex-specific guidelines for cardiovascular disease? J Am Coll Cardiol 2021;78:189-92.
3. Paz Ocaranza M, Riquelme JA, García L, et al. Counter-regulatory renin-angiotensin system in cardiovascular disease. Nat Rev Cardiol 2020;17:116-29.
4. Miller AJ, Arnold AC. The renin-angiotensin system and cardiovascular autonomic control in aging. Peptides 2022;150:170733.
5. Wang M, Khazan B, Lakatta EG. Central arterial aging and angiotensin II signaling. Curr Hypertens Rev 2010;6:266-81.
6. DuPont JJ, Kim SK, Kenney RM, Jaffe IZ. Sex differences in the time course and mechanisms of vascular and cardiac aging in mice: role of the smooth muscle cell mineralocorticoid receptor. Am J Physiol Heart Circ Physiol 2021;320:H169-80.
7. Berry C, Touyz R, Dominiczak AF, Webb RC, Johns DG. Angiotensin receptors: signaling, vascular pathophysiology, and interactions with ceramide. Am J Physiol Heart Circ Physiol 2001;281:H2337-65.
8. Peluso AA, Bertelsen JB, Andersen K, et al. Identification of protein phosphatase involvement in the AT2 receptor-induced activation of endothelial nitric oxide synthase. Clin Sci 2018;132:777-90.
9. Steckelings UM, Widdop RE, Sturrock ED, et al. The angiotensin AT2 receptor: from a binding site to a novel therapeutic target. Pharmacol Rev 2022;74:1051-135.
10. You D, Loufrani L, Baron C, Levy BI, Widdop RE, Henrion D. High blood pressure reduction reverses angiotensin II type 2 receptor-mediated vasoconstriction into vasodilation in spontaneously hypertensive rats. Circulation 2005;111:1006-11.
11. Sampson AK, Moritz KM, Jones ES, Flower RL, Widdop RE, Denton KM. Enhanced angiotensin II type 2 receptor mechanisms mediate decreases in arterial pressure attributable to chronic low-dose angiotensin II in female rats. Hypertension 2008;52:666-71.
12. Touyz RM, Endemann D, He G, Li JS, Schiffrin EL. Role of AT2 receptors in angiotensin II-stimulated contraction of small mesenteric arteries in young SHR. Hypertension 1999;33:366-72.
13. Viswanathan M, Tsutsumi K, Correa FM, Saavedra JM. Changes in expression of angiotensin receptor subtypes in the rat aorta during development. Biochem Biophys Res Commun 1991;179:1361-7.
14. Chatziantoniou C, Arendshorst WJ. Angiotensin receptor sites in renal vasculature of rats developing genetic hypertension. Am J Physiol 1993;265:F853-62.
15. Savoia C, Touyz RM, Volpe M, Schiffrin EL. Angiotensin type 2 receptor in resistance arteries of type 2 diabetic hypertensive patients. Hypertension 2007;49:341-6.
16. Lang JA, Krajek AC. Age-related differences in the cutaneous vascular response to exogenous angiotensin II. Am J Physiol Heart Circ Physiol 2019;316:H516-21.
17. Schwartz KS, Lang JA, Stanhewicz AE. Angiotensin II type 2 receptor-mediated dilation is greater in the cutaneous microvasculature of premenopausal women compared with men. J Appl Physiol 2023;135:1236-42.
18. Herrick AL, Batta R, Overbeck K, et al. A phase 2 trial investigating the effects of the angiotensin II type 2 receptor agonist C21 in systemic sclerosis-related Raynaud’s. Rheumatology 2023;62:824-8.
19. Tornling G, Batta R, Porter JC, et al. Seven days treatment with the angiotensin II type 2 receptor agonist C21 in hospitalized COVID-19 patients; a placebo-controlled randomised multi-centre double-blind phase 2 trial. EClinicalMedicine 2021;41:101152.
20. Xue B, Pamidimukkala J, Hay M. Sex differences in the development of angiotensin II-induced hypertension in conscious mice. Am J Physiol Heart Circ Physiol 2005;288:H2177-84.
21. Xue B, Pamidimukkala J, Lubahn DB, Hay M. Estrogen receptor-alpha mediates estrogen protection from angiotensin II-induced hypertension in conscious female mice. Am J Physiol Heart Circ Physiol 2007;292:H1770-6.
22. Tatchum-Talom R, Eyster KM, Martin DS. Sexual dimorphism in angiotensin II-induced hypertension and vascular alterations. Can J Physiol Pharmacol 2005;83:413-22.
23. Widdop RE, Vinh A, Henrion D, Jones ES. Vascular angiotensin AT2 receptors in hypertension and ageing. Clin Exp Pharmacol Physiol 2008;35:386-90.
24. Koike G, Horiuchi M, Yamada T, Szpirer C, Jacob HJ, Dzau VJ. Human type 2 angiotensin II receptor gene: cloned, mapped to the X chromosome, and its mRNA is expressed in the human lung. Biochem Biophys Res Commun 1994;203:1842-50.
25. Mishra JS, Hankins GD, Kumar S. Testosterone downregulates angiotensin II type-2 receptor via androgen receptor-mediated ERK1/2 MAP kinase pathway in rat aorta. J Renin Angiotensin Aldosterone Syst 2016;17:147032031667487.
26. Mishra JS, Te Riele GM, Qi QR, et al. Estrogen receptor-β mediates estradiol-induced pregnancy-specific uterine artery endothelial cell angiotensin type-2 receptor expression. Hypertension 2019;74:967-74.
27. Nelson JF, Felicio LS, Randall PK, Sims C, Finch CE. A longitudinal study of estrous cyclicity in aging C57BL/6J mice: I. Cycle frequency, length and vaginal cytology. Biol Reprod 1982;27:327-39.
28. Kim SK, McCurley AT, DuPont JJ, et al. Smooth muscle cell-mineralocorticoid receptor as a mediator of cardiovascular stiffness with aging. Hypertension 2018;71:609-21.
29. DuPont JJ, McCurley A, Davel AP, et al. Vascular mineralocorticoid receptor regulates microRNA-155 to promote vasoconstriction and rising blood pressure with aging. JCI Insight 2016;1:e88942.
30. DuPont JJ, Ramick MG, Farquhar WB, Townsend RR, Edwards DG. NADPH oxidase-derived reactive oxygen species contribute to impaired cutaneous microvascular function in chronic kidney disease. Am J Physiol Renal Physiol 2014;306:F1499-506.
31. Dupont JJ, Farquhar WB, Townsend RR, Edwards DG. Ascorbic acid or L-arginine improves cutaneous microvascular function in chronic kidney disease. J Appl Physiol 2011;111:1561-7.
32. Turner CG, Stanhewicz AE, Nielsen KE, Otis JS, Feresin RG, Wong BJ. Effects of biological sex and oral contraceptive pill use on cutaneous microvascular endothelial function and nitric oxide-dependent vasodilation in humans. J Appl Physiol 2023;134:858-67.
33. Turner CG, Hayat MJ, Grosch C, Quyyumi AA, Otis JS, Wong BJ. Endothelin a receptor inhibition increases nitric oxide-dependent vasodilation independent of superoxide in non-hispanic black young adults. J Appl Physiol 2023;134:891-9.
34. Reckelhoff JF. Mechanisms of sex and gender differences in hypertension. J Hum Hypertens 2023;37:596-601.
35. Mishra JS, Kumar S. Activation of angiotensin type 2 receptor attenuates testosterone-induced hypertension and uterine vascular resistance in pregnant rats. Biol Reprod 2021;105:192-203.
36. Widdop RE, Jones ES, Hannan RE, Gaspari TA. Angiotensin AT2 receptors: cardiovascular hope or hype? Br J Pharmacol 2003;140:809-24.
37. González-Blázquez R, Alcalá M, Fernández-Alfonso MS, et al. C21 preserves endothelial function in the thoracic aorta from DIO mice: role for AT2, Mas and B2 receptors. Clin Sci 2021;135:1145-63.
38. Pinaud F, Bocquet A, Dumont O, et al. Paradoxical role of angiotensin II type 2 receptors in resistance arteries of old rats. Hypertension 2007;50:96-102.
39. Waheed N, Elias-Smale S, Malas W, et al. Sex differences in non-obstructive coronary artery disease. Cardiovasc Res 2020;116:829-40.
40. Toering TJ, van der Graaf AM, Visser FW, et al. Gender differences in response to acute and chronic angiotensin II infusion: a translational approach. Physiol Rep 2015;3:e12434.
41. Brown RD, Hilliard LM, Head GA, Jones ES, Widdop RE, Denton KM. Sex differences in the pressor and tubuloglomerular feedback response to angiotensin II. Hypertension 2012;59:129-35.
42. Sampson AK, Hilliard LM, Moritz KM, et al. The arterial depressor response to chronic low-dose angiotensin II infusion in female rats is estrogen dependent. Am J Physiol Regul Integr Comp Physiol 2012;302:R159-65.
43. Barsha G, Mirabito Colafella KM, Walton SL, et al. In aged females, the enhanced pressor response to angiotensin II is attenuated by estrogen replacement via an angiotensin type 2 receptor-mediated mechanism. Hypertension 2021;78:128-37.
44. Dai SY, Peng W, Zhang YP, Li JD, Shen Y, Sun XF. Brain endogenous angiotensin II receptor type 2 (AT2-R) protects against DOCA/salt-induced hypertension in female rats. J Neuroinflammation 2015;12:47.
45. Hilliard LM, Nematbakhsh M, Kett MM, et al. Gender differences in pressure-natriuresis and renal autoregulation: role of the Angiotensin type 2 receptor. Hypertension 2011;57:275-82.
46. Mirabito KM, Hilliard LM, Kett MM, et al. Sex- and age-related differences in the chronic pressure-natriuresis relationship: role of the angiotensin type 2 receptor. Am J Physiol Renal Physiol 2014;307:F901-7.
47. Manrique-Acevedo C, Chinnakotla B, Padilla J, Martinez-Lemus LA, Gozal D. Obesity and cardiovascular disease in women. Int J Obes 2020;44:1210-26.
48. Lum-Naihe K, Toedebusch R, Mahmood A, et al. Cardiovascular disease progression in female zucker diabetic fatty rats occurs via unique mechanisms compared to males. Sci Rep 2017;7:17823.
49. Belenchia AM, Boukhalfa A, DeMarco VG, et al. Cardiovascular protective effects of NP-6A4, a drug with the FDA designation for pediatric cardiomyopathy, in female rats with obesity and pre-diabetes. Cells 2023;12:1373.
50. Gavini MP, Mahmood A, Belenchia AM, et al. Suppression of inflammatory cardiac cytokine network in rats with untreated obesity and pre-diabetes by AT2 receptor agonist NP-6A4. Front Pharmacol 2021;12:693167.
51. Baiardi G, Macova M, Armando I, Ando H, Tyurmin D, Saavedra JM. Estrogen upregulates renal angiotensin II AT1 and AT2 receptors in the rat. Regul Pept 2005;124:7-17.
52. Macova M, Armando I, Zhou J, et al. Estrogen reduces aldosterone, upregulates adrenal angiotensin II AT2 receptors and normalizes adrenomedullary Fra-2 in ovariectomized rats. Neuroendocrinology 2008;88:276-86.
53. Stewart JM, Taneja I, Raghunath N, Clarke D, Medow MS. Intradermal angiotensin II administration attenuates the local cutaneous vasodilator heating response. Am J Physiol Heart Circ Physiol 2008;295:H327-34.
54. Barsha G, Denton KM, Mirabito Colafella KM. Sex- and age-related differences in arterial pressure and albuminuria in mice. Biol Sex Differ 2016;7:57.
Cite This Article
How to Cite
Turner, C. G.; de Oliveira K.; Lu Q.; Patel A. R.; Pulakat L.; Jaffe I. Z.; DuPont J. J. Microvascular angiotensin II type 2 receptor function is enhanced in young females and declines in a model of murine aging. J. Cardiovasc. Aging. 2024, 4, 19. http://dx.doi.org/10.20517/jca.2024.09
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Data & Comments
Data






















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.