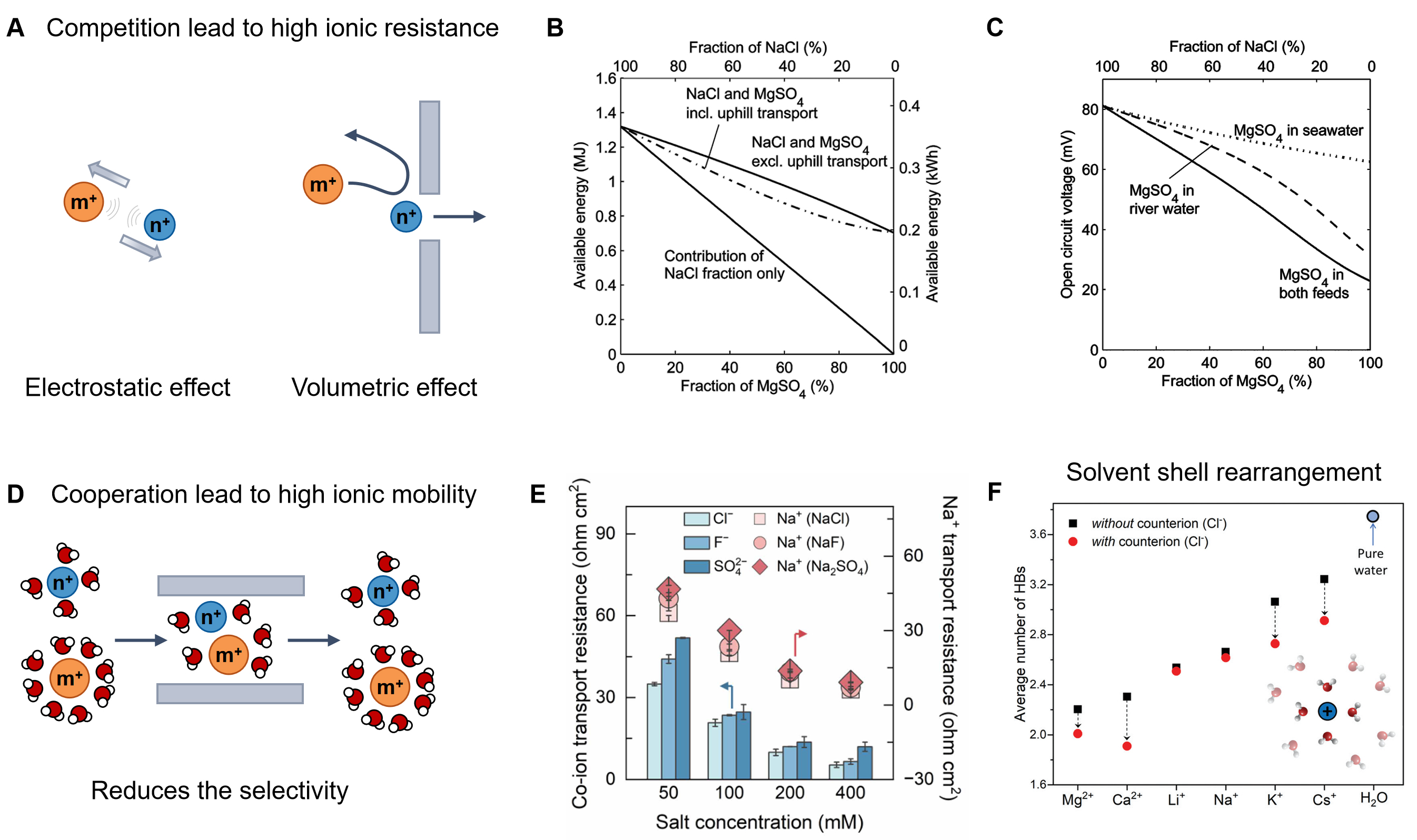
fig2
Figure 2. Interactions between ions. (A) Schematic of the ion-ion competitive interactions; (B) The osmotic power and (C) Open circuit voltage in NaCl varies with the concentration of divalent Mg2+ salt. Those figures are quoted with permission from the Royal Society of Chemistry[13]; (D) Schematic of the ion-ion cooperative interactions; (E) Ion transport resistance with increasing salt concentration. This figure is quoted with permission from the American Association for the Advancement of Science[38]; (F) Average number of hydrogen bonds (HBs) in the first hydration shell of metal ions with and without the counterions. This figure is quoted with permission from the Royal Society of Chemistry[42].