fig7
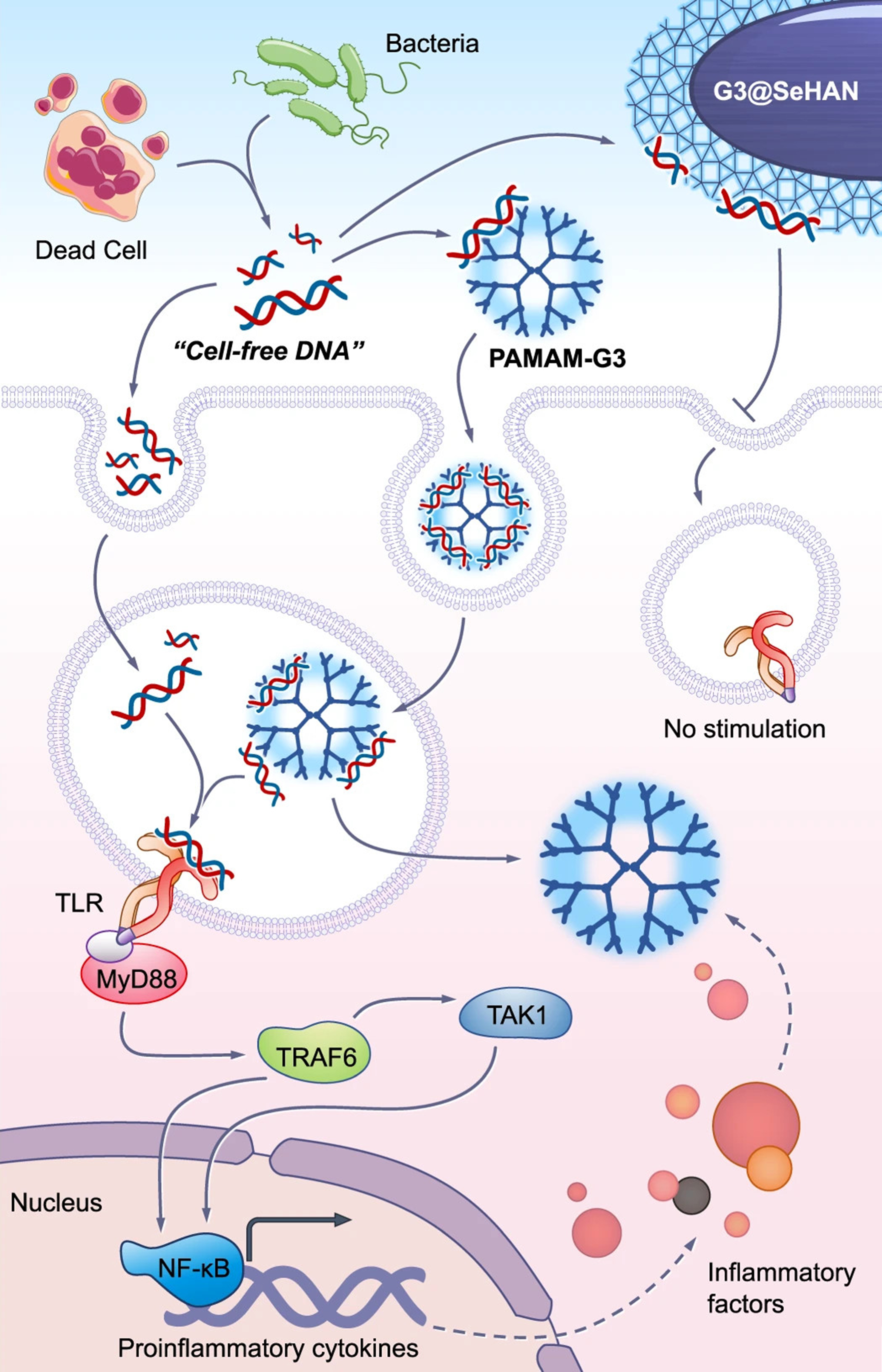
Figure 7. cfDNA-scavenging mechanisms of G3@SeHANs and PAMAM-G3 for the detection of periodontitis. cfDNA released from dead cells or bacteria is present in the extracellular environment and can be internalized by immune cells through endocytosis. Free cfDNA can traffic to endosomal compartments, where it engages TLRs, leading to recruitment of MyD88 and downstream activation of TRAF6 and TAK1. This signaling cascade culminates in nuclear translocation of NF-κB and the induction of pro-inflammatory cytokines and inflammatory mediators. PAMAM-G3 binds cfDNA and is internalized into endosomes; however, the cfDNA–PAMAM-G3 complexes remain capable of stimulating endosomal TLR signaling, thereby sustaining inflammatory responses. In contrast, G3@SeHANs efficiently scavenge cfDNA in the extracellular space and prevent its productive interaction with endosomal TLRs following cellular uptake. By sequestering cfDNA and blocking TLR–MyD88 signaling, G3@SeHANs suppress downstream activation of TRAF6, TAK1, and NF-κB, resulting in reduced transcription of pro-inflammatory cytokines and attenuation of inflammatory factor release. Reproduced with permission from[230]. cfDNA: Cell-free DNA; G3@SeHANs: generation 3 selenium–heparin-based anionic nanostructures; PAMAM-G3: poly(amidoamine) generation 3 dendrimers; TLRs: Toll-like receptors; MyD88: myeloid differentiation primary response protein 88; TRAF6: tumor necrosis factor receptor–associated factor 6; TAK1: transforming growth factor beta–activated kinase 1; NF-κB: nuclear factor kappa B.