fig8
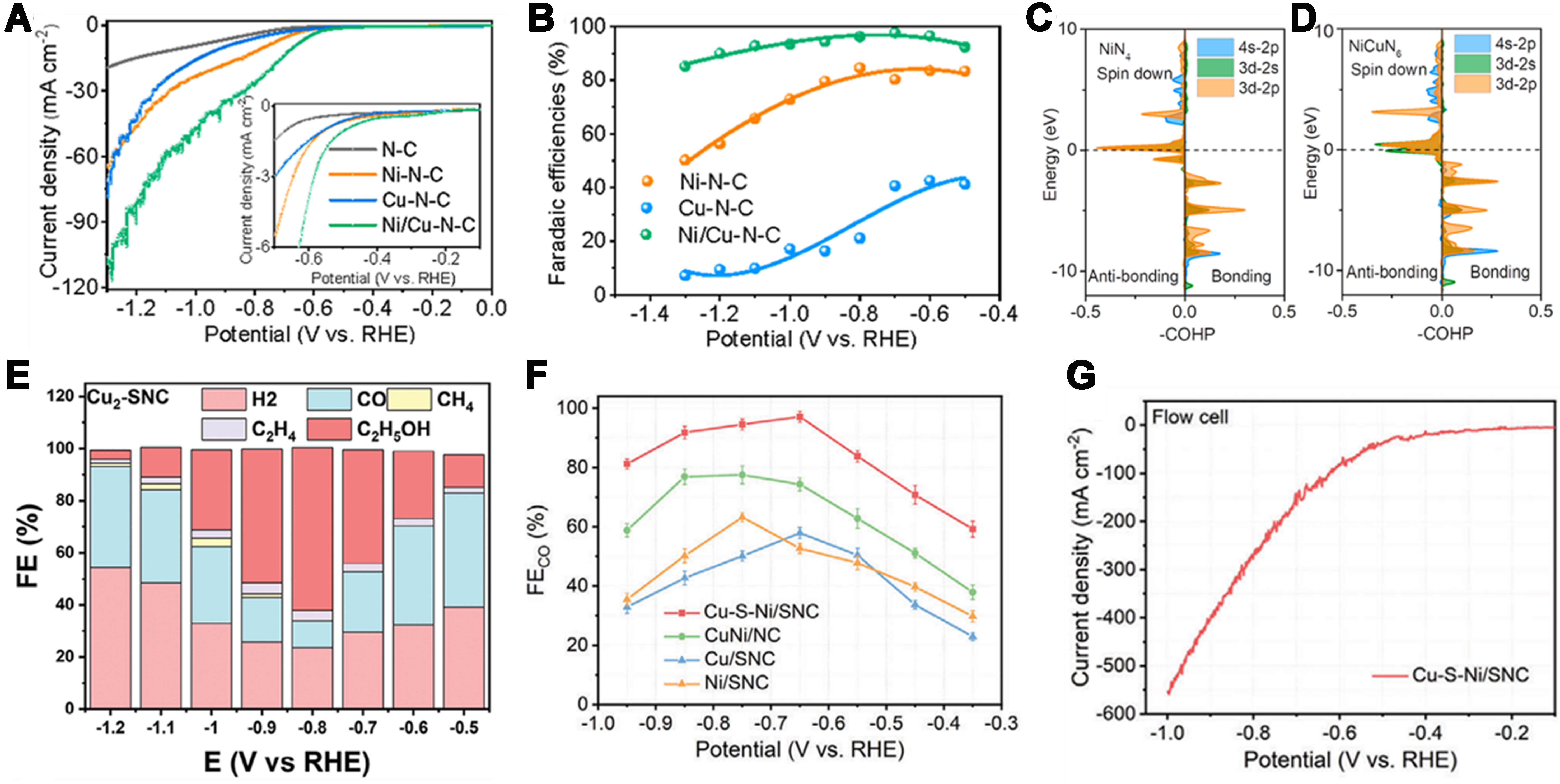
Figure 8. (A) LSV curves acquired in CO2-saturated 0.5 M KHCO3 solution on a rotating disc electrode at a rotating speed of 1,600 rpm. The inset highlights the LSV curves in the potential range from -0.1 to -0.7 V; (B) FE for CO production at various applied potentials; (C and D) COHP analysis of the Ni–C bond on NiN4 and NiCuN6; note that -COHP is used as the a-axis[69]. Copyright 2022, American Chemical Society; (E) FE value at different potentials by Cu2-SNC catalysts[96]. Copyright 2024, Wiley-VCH; (F) FECO at different potentials of Cu-S-Ni/SNC and references; (G) LSV curves of Cu-S-Ni/SNC in the flow cell[75]. Copyright 2024, Wiley-VCH. LSV: Linear sweep voltammetry; FE: Faradaic efficiency; COHP: crystal orbital Hamilton population; SNC: sulfur- and nitrogen-co-doped carbon.