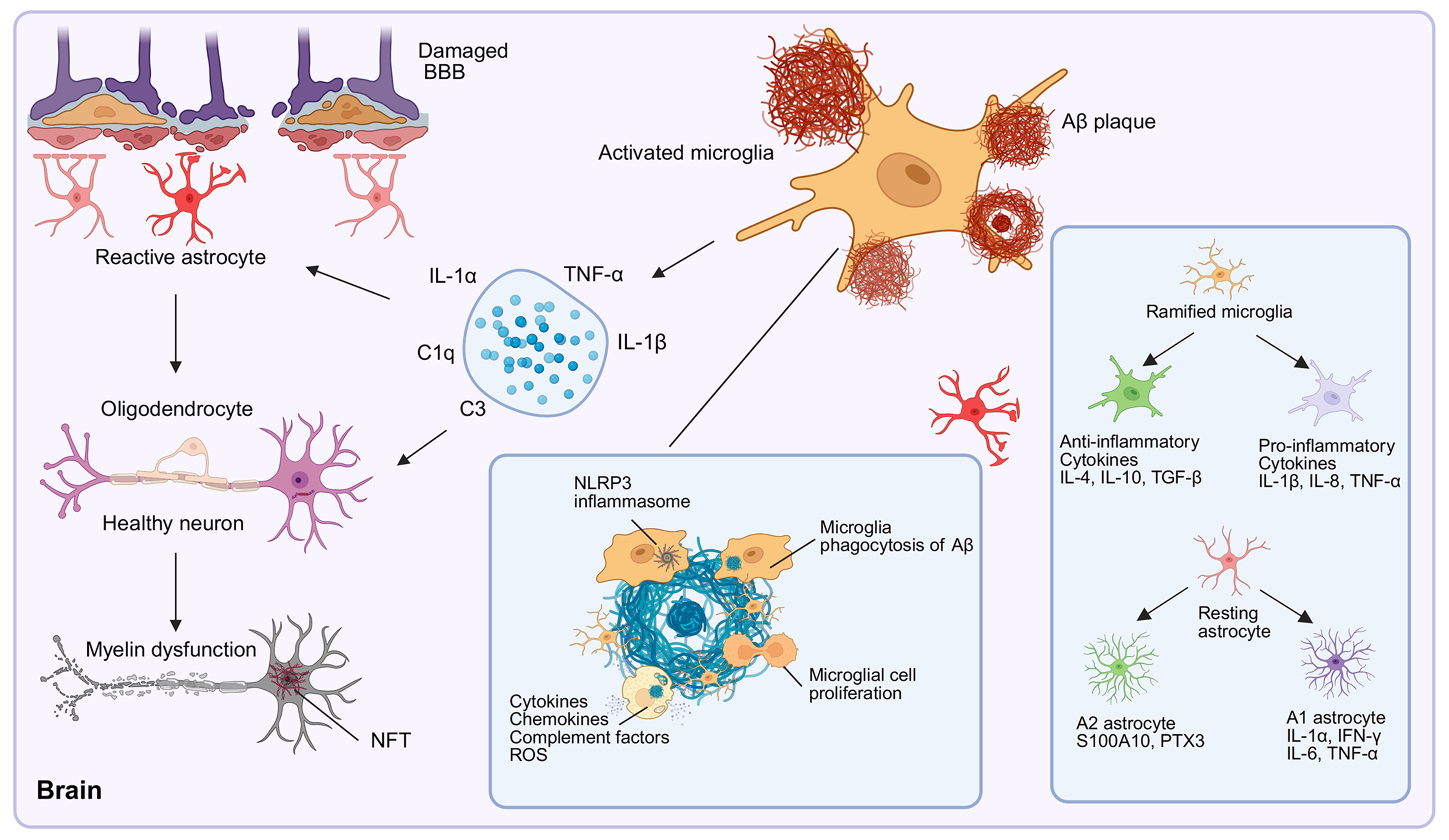
fig3
Figure 3. Role of glial cells in AD pathogenesis. Microglia transition from a ramified morphology to an activated state in an attempt to phagocytose Aβ. Chronic activation prompts microglia to release proinflammatory cytokines, ROS, and complement factors. This neuroinflammation promotes astrocytic reactivity, driving their polarization into proinflammatory A1 or potentially anti-inflammatory A2 subtypes, thereby contributing to astrogliosis. The interplay among activated glia, Aβ plaques, NFTs, BBB disruption, and inflammatory mediators (including those derived from the NLRP3 inflammasome) drives neuronal damage and disease progression, highlighting the dual neuroprotective and neurotoxic roles of glial cells in AD pathogenesis. [Created in BioRender. 1, 1. (2025) https://BioRender.com/w3zqhhf]. AD: Alzheimer’s disease; Aβ: amyloid-β; ROS: reactive oxygen species; NFT: neurofibrillary tangle; NFTs: neurofibrillary tangles; BBB: blood-brain barrier; NLR: NOD-like receptor; NLRP3: the NLR family pyrin domain containing 3.