EndoFLIP-guided foregut surgery: toward a new era of intraoperative physiology
Abstract
The endoluminal functional lumen imaging probe (EndoFLIP) has emerged as a transformative tool in the operative and perioperative management of foregut disorders, including achalasia, gastroesophageal reflux disease (GERD), and esophagogastric junction outflow obstruction. Unlike traditional modalities such as high-resolution manometry and barium swallow studies, EndoFLIP provides real-time, intraoperative assessment of distensibility, compliance, and luminal geometry at the esophagogastric junction. As such, EndoFLIP may augment surgical precision in procedures such as Heller myotomy, fundoplication, and peroral endoscopic myotomy (POEM) by enabling physiologic calibration rather than reliance on static anatomical correction. We further discuss its evolving role in risk stratification, postoperative surveillance, and personalized foregut surgical planning. As the field moves toward functional and minimally invasive solutions, EndoFLIP may offer a data-driven framework to optimize patient outcomes through physiology-informed decision-making in real time.
Keywords
INTRODUCTION
Foregut disorders such as achalasia, gastroesophageal reflux disease (GERD), and esophagogastric junction outflow obstruction (EGJOO) are commonly evaluated using esophagogastroduodenoscopy (EGD), high-resolution manometry (HRM), and barium swallow studies. While these modalities inform esophageal structure and motility, they provide limited real-time assessment of dynamic biomechanical properties such as compliance and distensibility[1,2].
The endoluminal functional lumen imaging probe (EndoFLIP) directly quantifies esophagogastric junction (EGJ) biomechanics using impedance planimetry within a fluid-filled balloon to measure cross-sectional area (CSA) and pressure, generating the distensibility index (DI)[3,4]. Unlike HRM, which measures pressure gradients and primary peristalsis, EndoFLIP evaluates EGJ expansion under load and secondary peristalsis, key physiological features in obstructive or relaxation disorders.
EndoFLIP was developed in response to the clinical limitations of conventional testing, particularly in patients with equivocal HRM findings such as type III achalasia or EGJOO, where decisions regarding myotomy remain challenging[2]. Postoperative complications, including dysphagia after fundoplication or myotomy, further highlight the need for real-time physiological guidance beyond static anatomy[2,5,6].
Accordingly, EndoFLIP complements manometry by providing dynamic, intraoperative physiological data that may improve diagnostic accuracy, guide surgical decision-making, and personalize therapy, reflecting a broader shift toward precision physiology in foregut surgery[7,8].
THE EVOLUTION OF SURGICAL DECISION-MAKING IN THE FOREGUT: WHY ENDOFLIP MATTERS INTRAOPERATIVELY
Traditional limitations in foregut surgery
Decision-making in surgery for foregut disorders has traditionally depended on a mix of preoperative evaluations and intraoperative anatomical observations. Nevertheless, several surgical techniques - including endoscopic and surgical myotomy as well as fundoplication - address structural concerns and functional problems, particularly regarding the EGJ. Standard methods such as HRM, upper endoscopy, and radiologic imaging offer valuable insights into luminal function[1,2].
However, these metrics are challenging to apply both intraoperatively and postoperatively, making it difficult to integrate findings and identify the causes of persistent symptoms - raising concerns about the adequacy of surgical intervention[2,9]. This has exposed a clinical grey area, revealing that anatomical corrections do not always ensure functional improvement, highlighting the inadequacies of strictly focusing on structural measures.
EndoFLIP as a physiological feedback tool
EndoFLIP addresses this gap by allowing real-time intraoperative assessment of EGJ compliance, distensibility, and diameter. The catheter is inserted trans orally and inflated with conductive saline within a compliant balloon that gauges both CSA and intrabag pressure across various segments, subsequently calculating the DI in mm2/mmHg[3]. This information is especially pertinent in procedures focused on adjusting EGJ tone.
For instance, during laparoscopic Heller myotomy, intraoperative EndoFLIP can detect insufficient or excessive disruption of the lower esophageal sphincter (LES) that might lead to postoperative reflux. Pandolfino et al. revealed that a final DI > 3 mm2/mmHg correlated with favorable outcomes and reduced symptom recurrence in achalasia patients undergoing peroral endoscopic myotomy (POEM) and myotomy[3].
How EndoFLIP differs from manometry
In fundoplication, where the “tightness” of the wrap can be somewhat subjective and depend on the operator, EndoFLIP provides a physiologic measure of how the wrap affects EGJ compliance[4]. However, the optimal post-fundoplication DI threshold remains controversial. Some studies have suggested that very low DI values (< 0.9 mm2/mmHg) are associated with an increased risk of postoperative dysphagia, while others have reported higher thresholds for concern. Prior single-center series, such as those from Alkhatib et al., have found that post-fundoplication DI values below 1.8 mm2/mmHg correlate with higher rates of dysphagia, whereas values in the 2-3 mm2/mmHg range are associated with improved symptoms without compromising reflux control[5]. Nonetheless, these data are derived largely from a single institution that has progressively transitioned from complete to partial fundoplication over time, and even within these reports, the benefits appear to be most pronounced at intermediate follow-up (e.g., two years) but less consistent at later intervals. Thus, while intraoperative EndoFLIP can inform calibration during fundoplication, further multi-center validation and long-term follow-up are needed before firm conclusions can be drawn.
Guiding real-time decision-making across diverse pathologies
Beyond achalasia and GERD, EndoFLIP provides actionable intraoperative insight across multiple foregut disorders. In GERD, it enables assessment of EGJ distensibility during fundoplication, helping tailor wrap tightness to balance reflux control and dysphagia risk, while identifying hypotensive or transient LES dysfunction not captured by manometry[6]. In EGJOO, EndoFLIP helps differentiate true mechanical obstruction from transient spasm when manometry is equivocal[1]. During POEM for jackhammer esophagus and other spastic motility disorders, endoscopic functional lumen imaging probe (FLIP) panometry guides the extent of myotomy by visualizing real-time contractile patterns[8]. In reoperative foregut surgery, EndoFLIP provides reliable anatomical and functional assessment despite altered anatomy, without extensive dissection[7].
ENDOFLIP IN PRACTICE: BALLOON VOLUME CALIBRATION AND INTRAOPERATIVE FUNCTIONAL FEEDBACK
The role of balloon volume in physiologic assessment
A significant benefit of EndoFLIP is its capability to deliver real-time, graded evaluations of EGJ distensibility with various balloon volumes. The balloon, typically inflated with 30, 40, or 50 mL of saline, is equipped with multiple impedance sensors that create pressure-diameter profiles of the EGJ. This enables the calculation of the DI (mm2/mmHg), which is essential for assessing luminal compliance[9].
Using larger balloon volumes further distends the lumen, providing insights into the tissue’s elastic properties and the functional outcomes of therapeutic procedures such as myotomy or fundoplication. The application of graded balloon volumes allows healthcare professionals to evaluate baseline compliance, reserve capacity, and the dynamic responses of the EGJ under varying strain[10].
Application of EndoFLIP in GERD surgery
EndoFLIP has also demonstrated significant value in the management of GERD, particularly during fundoplication and hiatal hernia repair. Lee et al. have reported on how EndoFLIP can calibrate the EGJ intraoperatively, offering objective measurements of distensibility to guide the degree of fundoplication tightness[11]. These studies show that excessively low DI values (< 0.9-1.8 mm2/mmHg) predict a higher risk of postoperative dysphagia, while excessively high values (> 3.0-4.0 mm2/mmHg) may predispose patients to reflux recurrence[12]. By providing real-time functional data, EndoFLIP allows surgeons to tailor anti-reflux surgery to the individual patient, moving beyond a one-size-fits-all approach. Integrating EndoFLIP into GERD procedures thus enhances precision, reduces variability in outcomes, and provides a physiologic framework for optimizing symptom control[11,12].
Case-based interpretation: functional outcomes at varying balloon volumes
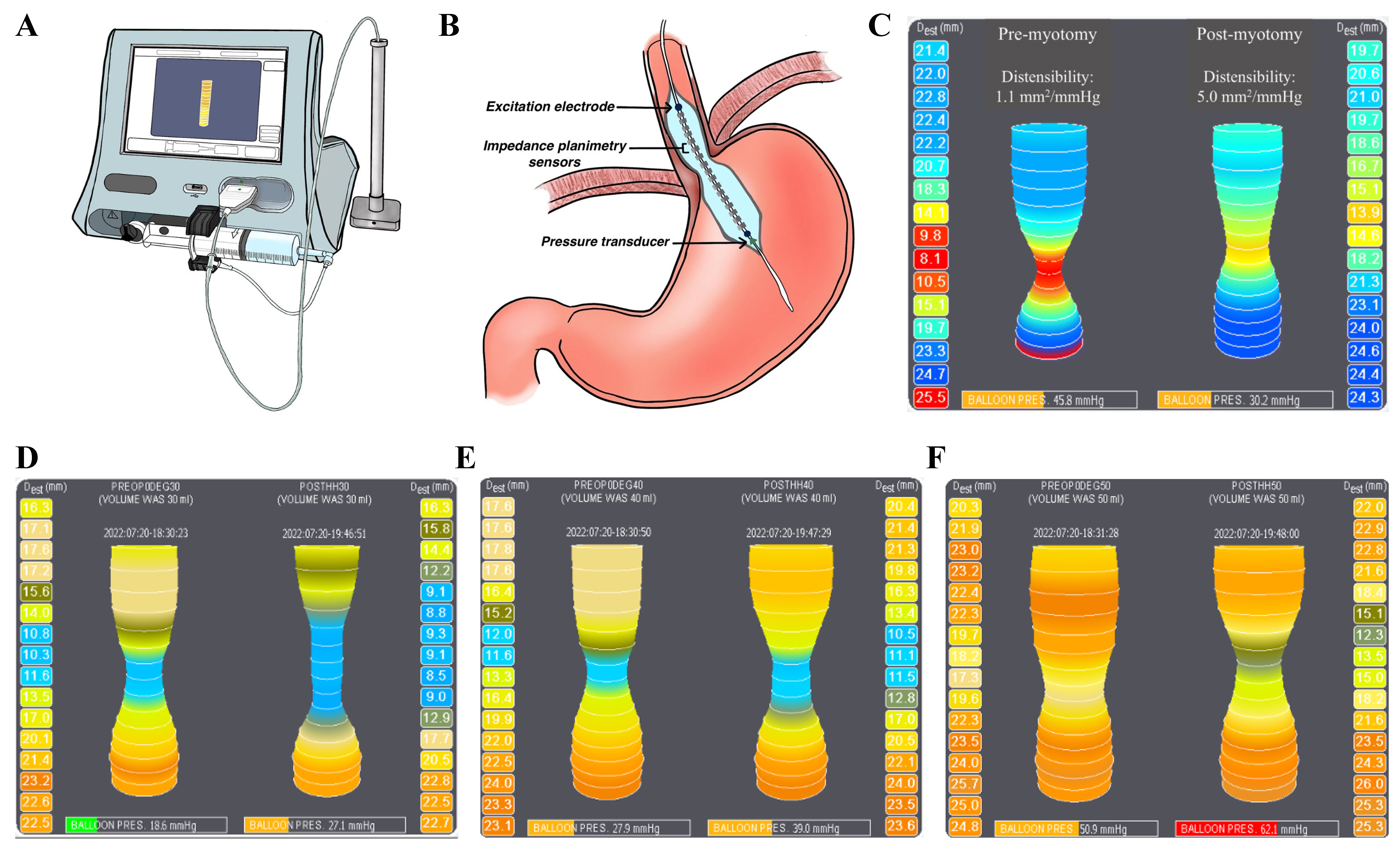
To facilitate interpretation of the patient-derived EndoFLIP recordings presented in subsequent figures, we include a schematic diagram of the device in situ. The EndoFLIP catheter is a balloon-based impedance planimetry system that incorporates excitation electrodes, multiple impedance sensors to measure CSA, and a pressure transducer. These elements together allow the calculation of the DI (DI = CSA/Pressure), a physiologic parameter central to understanding esophageal function. This schematic in Figure 1 provides context for how intraoperative EndoFLIP measurements are acquired and interpreted[13].
Figure 1. EndoFLIP system and intraoperative assessment of EGJ distensibility. The EndoFLIP console and catheter (A), EGJ catheter placement schematic (B), and representative achalasia tracing demonstrating an increase in DI from 1.1 to 5.0 mm2/mmHg after myotomy (C); Intraoperative topography at 30, 40, and 50 mL (D-F) shows volume-dependent changes in EGJ geometry and pressure, with postoperative findings consistent with symptom relief thresholds and safe distension profiles[14-18], supporting multi-volume functional assessment[19]. (A and B) adapted from Ref.[13]. (C-F) original. Figure assembled in BioRender. Turaga, A. (2026) https://BioRender.com/zihmyto. EndoFLIP: Endoluminal functional lumen imaging probe; EGJ: esophagogastric junction; DI: distensibility index.
Clinical utility of multi-volume assessment
Using various balloon volumes in sequence enables surgeons to assess both baseline and reserve function of the EGJ. For instance, a patient might show a slight increase in DI at 30 mL but a much greater improvement at 50 mL, indicating a structurally sound yet stiff EGJ that reacts positively to targeted disruption. On the other hand, no improvement over the different volumes could imply the necessity for a longer or more distal myotomy, or possibly a different diagnosis altogether[20].
Additionally, research indicates that post-myotomy DI thresholds are linked to outcomes. Jung suggested that a post-procedure DI exceeding 2.8 mm2/mmHg at 40 mL is associated with decreased dysphagia, while figures below 2.0 mm2/mmHg could indicate ongoing obstruction[21]. Likewise, Carducci et al. showed that utilizing intraoperative EndoFLIP with multiple balloon volumes facilitates the adjustment of the intervention, reducing the likelihood of under-treatment and over-treatment[22].
PROGNOSTIC AND PREDICTIVE POTENTIAL: TOWARD A PERSONALIZED FOREGUT SURGICAL APPROACH
Moving beyond intraoperative utility
EndoFLIP’s intraoperative use offers immediate insights into the effectiveness of interventions, but its predictive capabilities also reach into the preoperative and postoperative phases. By categorizing patients based on initial EGJ compliance and post-treatment distensibility, clinicians can anticipate symptom relief, recognize non-responders, and shape long-term management strategies.
Various studies indicate that certain DI thresholds are linked to postoperative results. A DI threshold greater than 2.8 mm2/mmHg, derived from prospective achalasia cohorts[14,15], is generally associated with symptom relief after myotomy. This presents a chance to transition from reactive, symptom-focused choices to proactive, physiology-based surgical planning.
EndoFLIP-guided risk stratification and procedure selection in achalasia
In the preoperative context, EndoFLIP offers distinct benefits compared to relying solely on manometry and impedance studies. For instance, in cases of EGJOO - where manometric results may resemble those of early achalasia or functional dysphagia - FLIP panometry effectively differentiates between actual mechanical obstruction (characterized by a low DI and high pressure) and dynamic or spastic variants[16,20]. In patients with Jackhammer esophagus or diffuse esophageal spasm - conditions that often result in varied symptom severity and treatment approaches - FLIP can illustrate repetitive and exaggerated contractile patterns, assisting in deciding the necessary length for myotomy or whether any intervention is warranted[16].
Guiding postoperative expectations and interventions
Postoperative EndoFLIP measurements are crucial in customizing follow-up strategies and guiding further interventions. A low DI following myotomy may indicate inadequate disruption of the EGJ, prompting the need for early re-intervention or pharmacologic support. In contrast, a high DI could indicate excessive opening, which may result in reflux and affect the decision regarding fundoplication augmentation[19]. Hirano et al. have suggested incorporating EndoFLIP metrics into postoperative surveillance protocols, especially in complex or reoperative foregut cases where symptoms continue despite previous treatments[23]. However, these measurements must ultimately be interpreted in conjunction with clinical symptoms, endoscopic findings, imaging studies, and preoperative pH testing to ensure accurate assessment and management.
The emerging role of machine learning and predictive models
Recent research has examined the integration of EndoFLIP-derived metrics into machine learning models for predicting outcomes. One study reported that artificial intelligence algorithms trained on FLIP panometry achieved classification accuracies exceeding 90% compared with expert interpretation, highlighting its potential for automated diagnosis[19]. Another large-scale analysis of more than 700 patients demonstrated that FLIP-based clustering could generate reproducible motility phenotypes with predictive value for postoperative outcomes[24].
Limitations of EndoFLIP
Despite its advantages, EndoFLIP has several limitations. Adoption is limited by cost and availability, as specialized equipment is not universally accessible[7]. Interpretation requires experience, and the lack of standardized training may introduce inter-operator variability. Although large panometry datasets have begun to define normative values[25], consensus guidelines remain limited, with thresholds varying by balloon volume, technique, and patient population.
Most outcome data linking EndoFLIP metrics to clinical benefit derive from single-center or retrospective studies, limiting generalizability[6,20]. Prospective, multicenter studies are needed to validate DI thresholds across diverse foregut disorders. Additionally, EndoFLIP measures luminal distensibility but does not assess peristaltic coordination, necessitating integration with manometry and other complementary modalities.
CONCLUSION
The EndoFLIP represents a shift from anatomy-based to function-focused foregut surgery by enabling real-time assessment of EGJ distensibility, compliance, and geometry. Across multiple balloon volumes, EndoFLIP provides objective confirmation of procedural effectiveness, supports tailored interventions, and may reduce postoperative complications such as dysphagia.
Beyond intraoperative use, EndoFLIP serves as a predictive and prognostic tool that informs personalized surgical planning, with established DI benchmarks increasingly incorporated into clinical decision-making. Integration of FLIP metrics into machine learning and predictive models further advances precision surgery by enabling data-driven customization.
Broader adoption will require standardized training, consensus normative values, and inclusion in clinical guidelines, supported by longitudinal and prospective validation studies. As gastrointestinal surgery evolves toward personalized, outcome-driven care, EndoFLIP has the potential to function as a physiologic guide, redefining surgical success through real-time functional restoration.
DECLARATIONS
Authors’ contributions
Made substantial contributions to the conception and design of the commentary and performed literature review and interpretation: Turaga AH
Provided academic and administrative support: Zarnegar R
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
Zarnegar R serves as a consultant for Medtronic, Becton Dickinson, and Intuitive Surgical. Zarnegar R is an Editorial Board Member of Mini-invasive Surgery and the Guest Editor for the Topic “Foregut Disease: Minimally Invasive and Innovative Therapeutics - Series II”. Zarnegar R was not involved in any aspect of the editorial process for this manuscript, including reviewer selection, manuscript handling, or decision-making. Turaga AH declares no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2026.
REFERENCES
1. Carlson DA, Kahrilas PJ, Lin Z, et al. Evaluation of esophageal motility utilizing the functional lumen imaging probe. Am J Gastroenterol. 2016;111:1726-35.
2. Yadlapati R, Kahrilas PJ, Fox MR, et al. Esophageal motility disorders on high-resolution manometry: Chicago classification version 4.0©. Neurogastroenterol Motil. 2021;33:e14058.
3. Pandolfino JE, de Ruigh A, Nicodème F, Xiao Y, Boris L, Kahrilas PJ. Distensibility of the esophagogastric junction assessed with the functional lumen imaging probe (FLIP™) in achalasia patients. Neurogastroenterol Motil. 2013;25:496-501.
4. Ma O, Brar K, McCluskey S, Morris-Janzen D, Peabody J, Turner S. Long-term outcomes after per-oral endoscopic myotomy versus laparoscopic Heller myotomy in the treatment of achalasia: a systematic review and meta-analysis. Surg Endosc. 2025;39:5985-94.
5. Alkhatib H, Haas AJ, Kara AM, et al. Tailoring the wrap: intraoperative functional lumen imaging probe (FLIP) during hiatal hernia repair. Surg Endosc. 2024;38:3425-32.
6. Greenberg JA, Stefanova DI, Reyes FV, et al. Quantifying physiologic parameters of the gastroesophageal junction during re-operative anti-reflux surgery. Surg Endosc. 2022;36:7008-15.
7. Savarino E, di Pietro M, Bredenoord AJ, et al. Use of the functional lumen imaging probe in clinical esophagology. Am J Gastroenterol. 2020;115:1786-96.
8. Campagna RAJ, Carlson DA, Hungness ES, et al. Intraoperative assessment of esophageal motility using FLIP during myotomy for achalasia. Surg Endosc. 2020;34:2593-600.
9. Pandolfino JE, Kwiatek MA, Nealis T, Bulsiewicz W, Post J, Kahrilas PJ. Achalasia: a new clinically relevant classification by high-resolution manometry. Gastroenterology. 2008;135:1526-33.
10. Goong HJ, Hong SJ, Kim SH. Intraoperative use of a functional lumen imaging probe during peroral endoscopic myotomy in patients with achalasia: a single-institute experience and systematic review. PLoS One. 2020;15:e0234295.
11. Lee JM, Yoo IK, Kim E, Hong SP, Cho JY. The usefulness of the measurement of esophagogastric junction distensibility by EndoFLIP in the diagnosis of gastroesophageal reflux disease. Gut Liver. 2021;15:546-52.
12. Al Asadi H, Najah H, Li Y, et al. Determination of causes of post-operative dysphagia after anti-reflux surgery based on intra-operative planimetry. Surg Endosc. 2024;38:5623-33.
13. Su B, Dunst C, Gould J, et al. Experience-based expert consensus on the intra-operative usage of the EndoFLIP impedance planimetry system. Surg Endosc. 2021;35:2731-42.
14. Teitelbaum EN, Soper NJ, Pandolfino JE, et al. Esophagogastric junction distensibility measurements during Heller myotomy and POEM for achalasia predict postoperative symptomatic outcomes. Surg Endosc. 2015;29:522-8.
15. Hoppo T, McMahon BP, Witteman BP, et al. Functional lumen imaging probe to assess geometric changes in the esophagogastric junction following endolumenal fundoplication. J Gastrointest Surg. 2011;15:1112-20.
16. Carlson DA, Kou W, Lin Z, et al. Normal values of esophageal distensibility and distension-induced contractility measured by functional luminal imaging probe panometry. Clin Gastroenterol Hepatol. 2019;17:674-81.e1.
17. Stanton MB, Pandolfino JE, Simlote A, Kahrilas PJ, Carlson DA. The esophageal response to distension on functional lumen imaging probe panometry is minimally changed by conscious sedation in healthy asymptomatic subjects. J Neurogastroenterol Motil. 2025;31:45-53.
18. Carlson DA, Lin Z, Kahrilas PJ, et al. The functional lumen imaging probe detects esophageal contractility not observed with manometry in patients with achalasia. Gastroenterology. 2015;149:1742-51.
19. Carlson DA, Gyawali CP, Khan A, et al. Classifying esophageal motility by FLIP panometry: a study of 722 subjects with manometry. Am J Gastroenterol. 2021;116:2357-66.
20. Low EE, Yadlapati R. Utility of functional lumen imaging probe in the evaluation of esophageal conditions. Am J Gastroenterol. 2024;119:15-20.
21. Jung KW. The clinical usefulness of functional luminal imaging probe in esophageal dysmotility disorder. J Neurogastroenterol Motil. 2022;28:509-11.
22. Carducci JM, Chang AC, Lagisetty KH, Kwon R, Lin J, Reddy RM. The role of intraoperative functional lumen imaging in peroral endoscopic myotomy and laparoscopic Heller myotomy. Ann Thorac Surg Short Rep. 2025;3:918-23.
23. Hirano I, Pandolfino JE, Boeckxstaens GE. Functional lumen imaging probe for the management of esophageal disorders: expert review from the Clinical Practice Updates Committee of the AGA Institute. Clin Gastroenterol Hepatol. 2017;15:325-34.
24. Kou W, Soni P, Klug MW, et al. An artificial intelligence platform provides an accurate interpretation of esophageal motility from functional lumen imaging probe panometry studies. Neurogastroenterol Motil. 2023;35:e14549.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Topic
Copyright
Data & Comments
Data
















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.