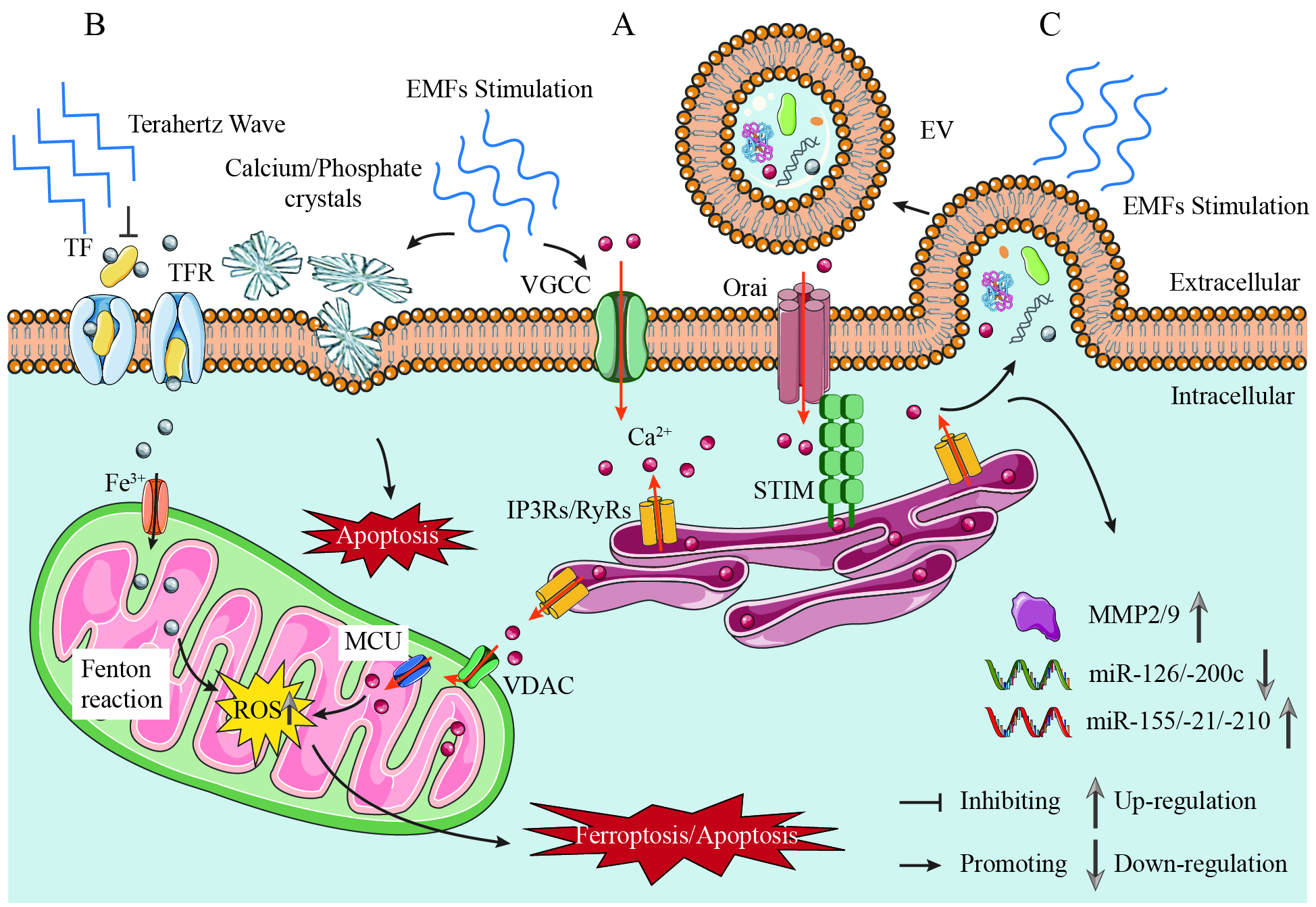
fig1
Figure 1. Mechanisms of EMF Regulation in Prostate Cancer: Calcium Signaling, Extracellular Vesicle Reprogramming, and Ferroptosis Modulation. (A) Calcium signaling as the central nodal effector. EMFs induce piezoelectric effects in prostatic calcium/phosphate crystals, generating mechanical stress that activates VGCCs, thereby initiating extracellular Ca2+ influx and intracellular signaling cascades, such as calcium-induced calcium release (CICR). EMFs (Low-Frequency) amplify this process, driving mitochondrial membrane depolarization and ROS overproduction, which synergizes with iron-dependent lipid peroxidation to potentiate ferroptosis; (B) Frequency-dependent ferroptosis modulation. EMFs (Low-Frequency) promote ferroptosis via the Ca2+-ROS-Fe2+ axis, inducing lipid peroxidation and oxidative damage. Terahertz Waves (34.5 THz) suppress ferroptosis by inhibiting iron-transferrin binding; (C)