Hierarchically porous two-dimensional Fe/N-codoped carbon nanoleaves for enhanced mass transfer and electrocatalytic oxygen reduction reaction
Abstract
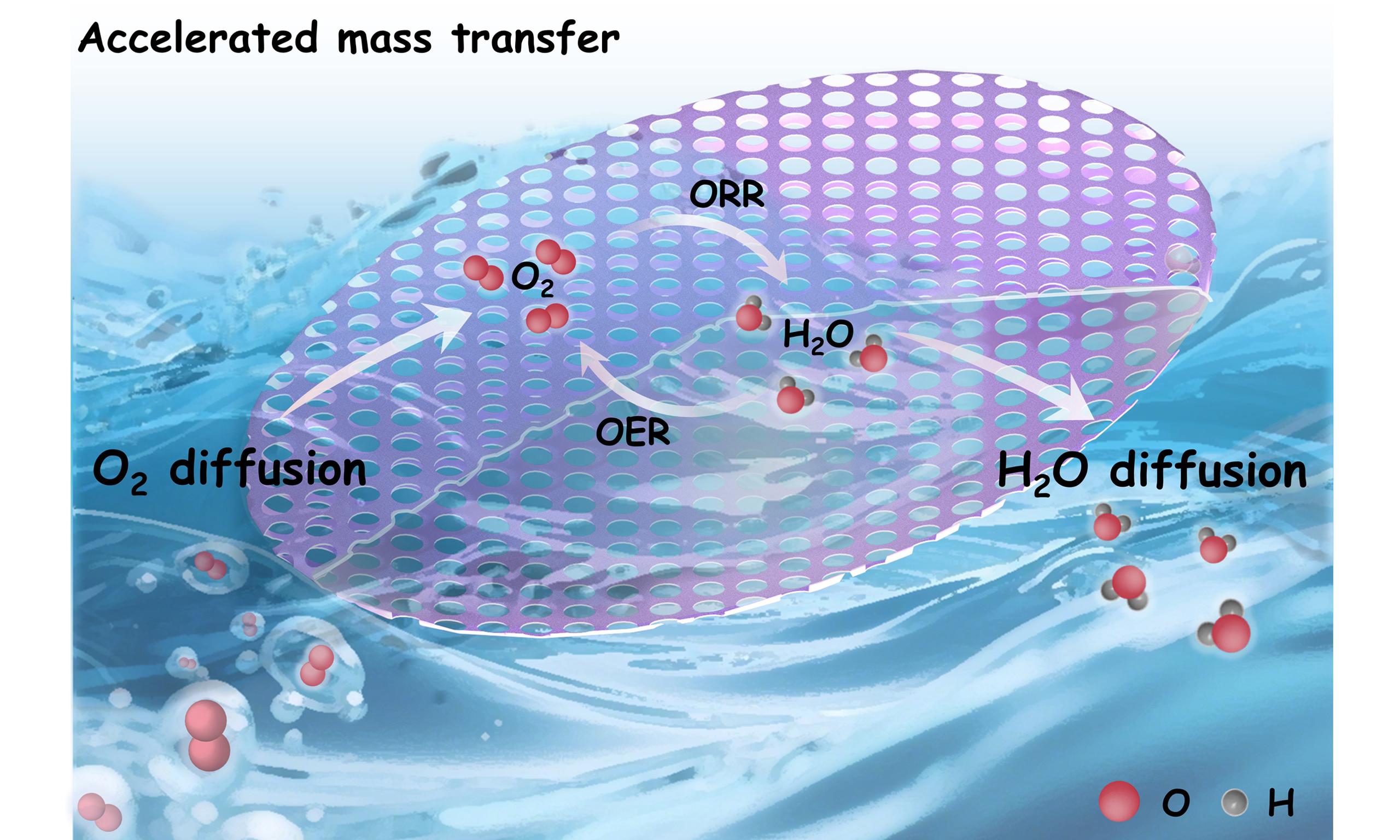
Zinc-air batteries are promising energy conversion devices with high theoretical energy density, but their practical performance is limited by the kinetically sluggish oxygen reduction reaction kinetics at the air electrode. This kinetic bottleneck stems from the inefficient mass transport and insufficient accessible active sites. In order to solve this problem, constructing porous structure at the air electrode could be an efficient strategy to improve mass transfer and expose more active sites. Herein, we successfully constructed hierarchical porous structure with mesopores and micropores in two-dimensional (2D) Fe/N-codoped carbon nanoleaves. F127 micelles on the surface were introduced for the formation of mesopores, while microporous structure came from 2D Fe-doped Zeolitic Imidazolate Framework-L (ZIF-L) precursors. After pyrolysis in Ar, the derived 2D meso/microporous Fe/N-codoped carbon nanoleaves possess atomically dispersed Fe-Nx sites. Kinetic experiments demonstrate that the hierarchical porous structure reduces the mass transfer resistance. Furthermore, density functional theory calculations reveal that the Fe-Nx active sites with concave curvature within the hierarchical porous
Keywords
INTRODUCTION
In recent years, rising global energy demand and growing environmental concerns have spurred extensive research into sustainable energy development and advanced storage technologies. To date, various clean energy sources (e.g., solar, wind, and tidal) and storage devices (e.g., fuel cells, lithium-ion, and metal-air batteries) have been developed[1-4]. Among these, zinc-air batteries (ZABs) have garnered scientific interest due to their low environmental impact, high energy density, and cost-effectiveness. Although traditional
In the mass transfer optimization of air cathode catalysts for ZABs, the construction of porous architectures has been demonstrated as an effective strategy to optimize oxygen diffusion pathways and enhance active site exposure[19-21]. Moreover, recent studies demonstrate that the concave curvature of porous channels can optimize the adsorption strength of key intermediates by inducing electronic structure distortion at active sites, thereby significantly enhancing the ORR kinetics[22]. Current pore-engineering strategies primarily include: hard templating techniques (e.g., ZnO/SiO2 sacrificial templates), etching approaches (e.g., KOH or plasma etching), and high-temperature pyrolysis-induced phase separation mechanisms[23,24]. For instance, Song et al. prepared FeCo/NC with 3D ordered hierarchical porous structure using a hard template [polystyrene (PS) microsphere] and inhomogeneous nucleation[25]. The unique 3D ordered hierarchical porous structure significantly optimizes the mass transfer kinetic pathway. Zhang et al. successfully constructed hierarchical pore systems through the pyrolysis of metal-organic framework (MOF) precursors, providing efficient mass transfer pathways for electrocatalytic CO2 reduction[26]. Recently, two-dimensional (2D) nanomaterials have become an attractive choice for pore engineering due to their high
Herein, we constructed an interconnected hierarchical porous structure with mesopores and micropores in 2D Fe/N-codoped carbon nanoleaves (Meso/Micro-FeNC). The mesopores on the surface were directed by F127/1,3,5-trimethylbenzene (TMB) micelles, while the micropores originated from 2D Fe-doped Zeolitic Imidazolate Framework-L (ZIF-L) precursors, resulting in an interconnected hierarchical porous architecture. The as-prepared Meso/Micro-FeNC features interconnected porous channels, a high surface area, and accessible Fe-Nx active sites. These characteristics improve mass transfer and provide more accessible active sites. Meanwhile, 2D hollow structure ensures mechanical robustness. Assembled ZABs delivered an open-circuit potential of 1.52 V with a remarkable specific capacity of 787.6 mAh g-1 at
EXPERIMENTAL
Synthesis of ZIF-L-Fe and ZIF-L
First, 0.594 g of Zn(NO3)2 and 5.2 mg of ferric ammonium citrate were dissolved in 40 mL of H2O to prepare Solution A. Separately, 1.314 g of 2-mim was dissolved in 40 mL of H2O to prepare Solution B. Subsequently, Solution B was rapidly poured into Solution A and stirred for 4 h at room temperature. The mixture was washed three times with ethanol and centrifuged at 8000 rpm for 5 min to obtain yellow
For the synthesis of ZIF-L, the same procedure was followed, omitting the addition of ferric ammonium citrate, ultimately yielding white ZIF-L product.
Synthesis of ZIF-L-Fe@F127 micelle
First, 0.1 g of F127 and 20 mg of dopamine (DA) were dissolved in a mixed solution of 5 mL H2O and 5 mL ethanol. The mixture was stirred slowly at 500 rpm for 30 min at room temperature. Then, 2 mL of TMB was added to form a microemulsion system, and stirring was continued at room temperature for 3 h until the system was stabilized. Subsequently, 100 mg of pre-synthesized ZIF-L-Fe was dispersed in a mixed solution of 10 mL H2O and 10 mL ethanol. This dispersion was gradually added into the previously prepared microemulsion system. The pH of the solution was adjusted to 8.5 using Tris-HCl buffer solution, and stirring was continued at 500 rpm for 48 h at room temperature. Finally, the mixture was centrifuged at 8000 rpm for 5 min and washed three times with ethanol and H2O to obtain ZIF-L-Fe@F127 micelle. The product was then dried in an oven at 60 oC for future use.
Synthesis of Meso/Micro-FeNC, FeNC, NC
ZIF-L-Fe@F127 micelle, ZIF-L-Fe, and ZIF-L samples were separately loaded into a tube furnace. The samples were heated at a rate of 2 oC min-1 to 350 oC and maintained at this temperature for 2 h under an Ar atmosphere. The temperature was elevated to 900 oC at the same rate and held for an additional 2 h. After cooling to room temperature, Meso/Micro-FeNC, Fe-NC, and NC were obtained.
RESULTS AND DISCUSSION
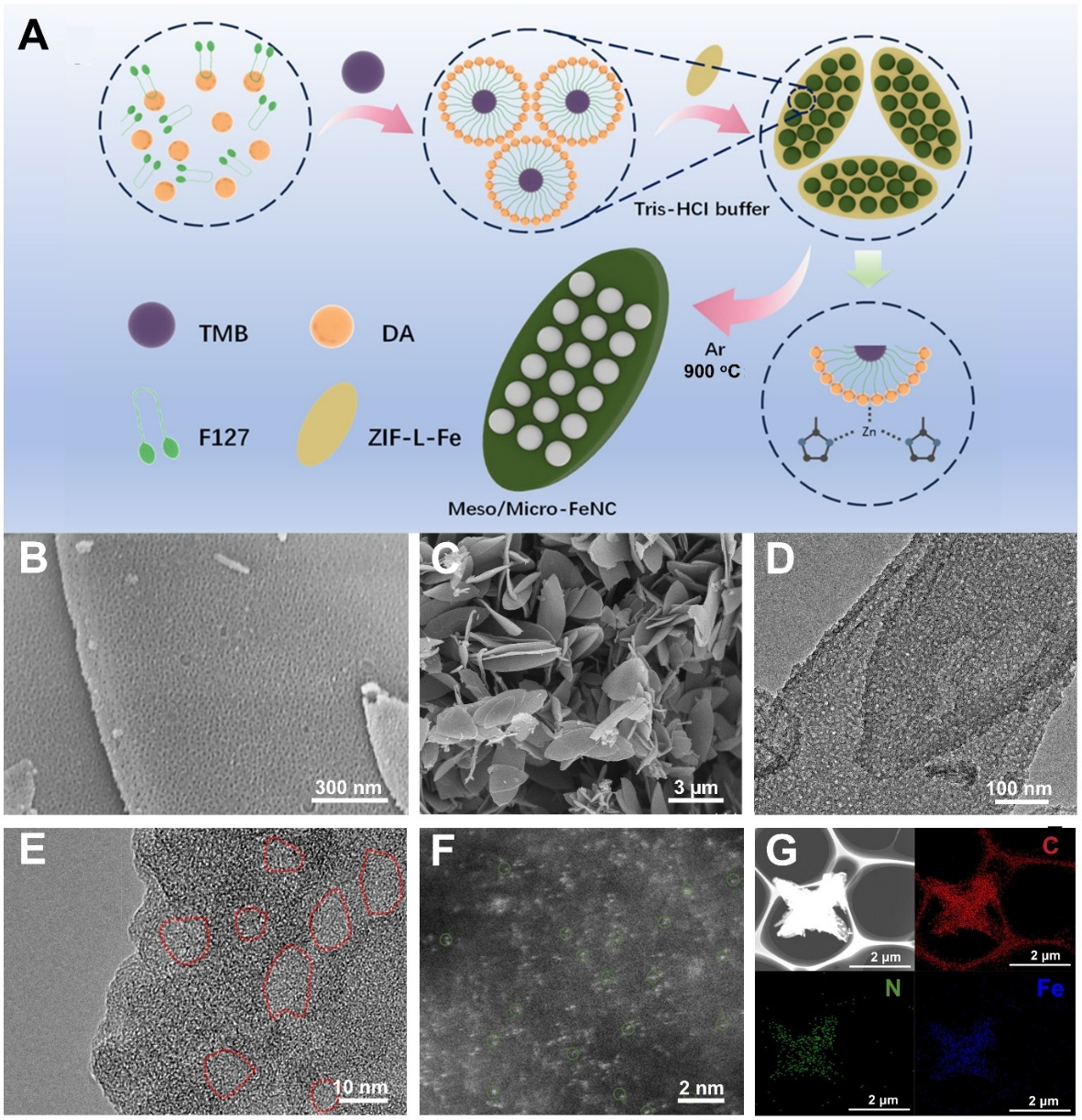
Figure 1A illustrates the synthesis of Meso/Micro-FeNC, which features single-atom Fe dispersed on interconnected hierarchical porous hollow nanoleaves. Typically, ZIF-L-Fe is pre-synthesized via a coordination-driven self-assembly strategy. This involves mixing aqueous solutions of 2-mim with zinc salts, and iron salts to construct the framework through metal-ligand coordination. For the iron source, we preferentially select ferric ammonium citrate, because the carbonyl group (C=O) in citrate anions can form strong coordination with Zn in the ZIF-L framework, partially replacing the original 2-mim ligands. This ligand exchange maintains the structural integrity of the ZIF crystals while establishing a Fe-Zn mixed coordination environment[38]. This environment facilitates Zn evaporation and the formation of Fe-Nx active sites during pyrolysis, without destroying the leaf-like architecture. Furthermore, active nitrogen species generated from the decomposition of ammonium ions during pyrolysis synergistically promote the formation of atomically dispersed Fe-Nx active sites. Compared to inorganic iron salts (e.g., FeCl3), ferric ammonium citrate offers superior aqueous solubility, which prevents active site aggregation or framework defects caused by hydrolysis-induced precipitation, while eliminating risks associated with residual corrosive anions. Subsequently, Pluronic F127, DA, and TMB are combined to assemble a DA layer on the surface of pre-synthesized ZIF-L-Fe (ZIF-L-Fe@micelle) via a nanoemulsion assembly strategy. Subsequently, a segmented pyrolysis protocol is applied. Initially, the surfactant is removed at a relatively low temperature, followed by high-temperature graphitization of the precursor. This two-step pyrolysis strategy effectively preserves the integrity of the pore structure, leading to the successful synthesis of
Figure 1. (A) The preparation process of Meso/Micro-FeNC; (B) SEM image of ZIF-L-Fe@micelle; (C) SEM and (D) TEM image of Meso/Micro-FeNC; (E) High resolution TEM image of Meso/Micro-FeNC (red circles: mesopores); (F) Aberration-corrected
As shown in the field-emission scanning electron microscopy (SEM) images in Figure 1B, the successful synthesis of the ZIF-L-Fe@micelle precursor is demonstrated, with ordered mesopores observed on its surface. After high-temperature pyrolysis process, the resulting Meso/Micro-FeNC retains the 2D leaf-like morphology of the precursor MOF [Figure 1C]. Transmission electron microscopy (TEM) image reveals a roughened surface composed of a continuous honeycomb-like mesoporous network and hollow cavities, with no metal nanoparticles [Figure 1D]. This hierarchical architecture provides more accessible active sites and enhances electrocatalytic mass transfer processes. High-resolution TEM image shows no iron nanoparticles or lattice fringes, confirming the high dispersion of iron species [Figure 1E]. Additionally, mesoporous structures with a pore size of approximately 6 nm (marked in red circles) were observed. Further insights into the atomic configuration of active sites were conducted using aberration-corrected high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM). The aberration-corrected HAADF-STEM image [Figure 1F] further reveals a high density of discrete bright spots (highlighted by green circles), directly demonstrating atomic-level Fe dispersion. Energy-dispersive
The structural properties of Meso/Micro-FeNC, Fe-NC, and NC were analyzed in depth using powder
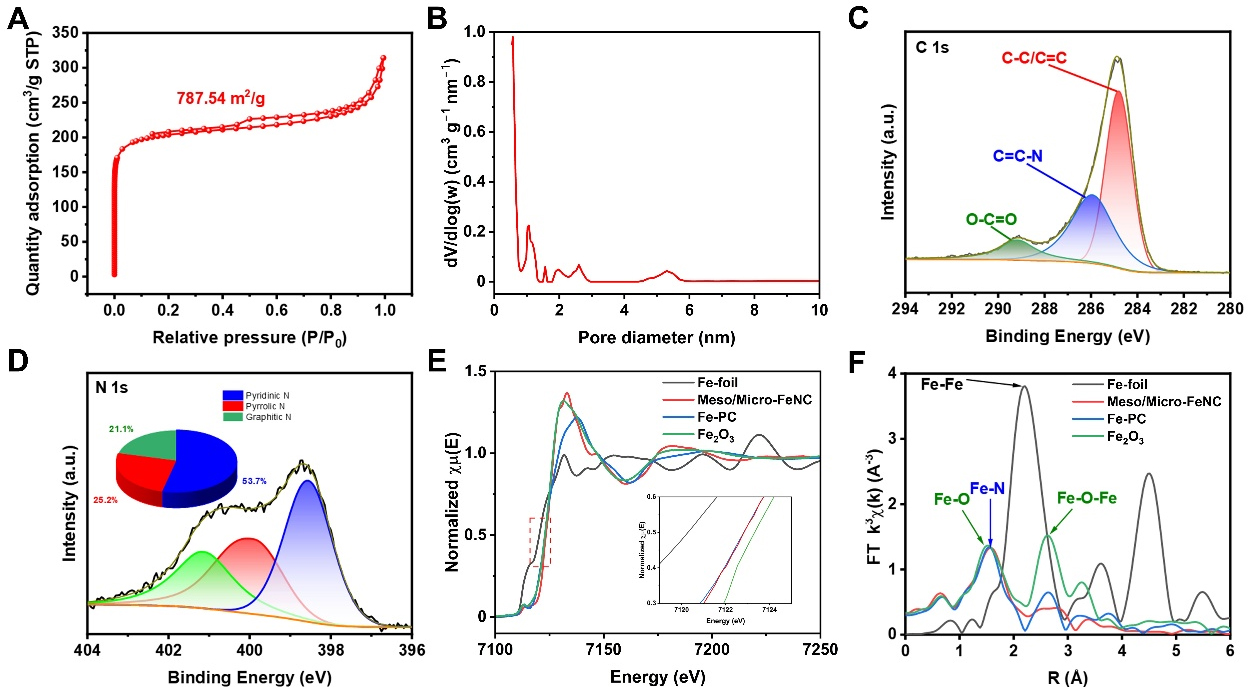
Figure 2. (A) N2 adsorption/desorption isotherms and (B) pore size distribution of Meso/Micro-FeNC; (C) XPS high-resolution spectra of C 1s and (D) N 1s for Meso/Micro-FeNC; (E) Fe K-edge XANES spectra and (F) FT k3-weighted EXAFS spectra of Fe foil, Meso/Micro-FeNC, FePc, and Fe2O3. Meso/Micro-FeNC: Meso/microporous Fe/N-codoped carbon; XPS: X-ray photoelectron spectroscopy; XANES: X-ray absorption near-edge structure.
The chemical states of the elements in Meso/Micro-FeNC and Fe-NC were further analyzed by X-ray photoelectron spectroscopy (XPS) [Supplementary Figure 9]. The results indicate that C, N, and O are present in the spectra of both Meso/Micro-FeNC and Fe-NC. As predicted, the Fe element is not detected in the survey scan spectra due to its relatively low content. The C 1s high-resolution spectrum shows three peaks assigned to O-C=O at 289.2 eV, C=C-N at 286.0 eV, and C=C/C-C at 284.8 eV [Figure 2C]. The N 1s high-resolution spectrum indicates three N forms in Meso/Miro-FeNC: pyrrolic-N at 398.6 eV, pyridinic-N at 400.1 eV, and graphitic-N at 401.2 eV [Figure 2D][43]. These results confirm the high density of N sites in Meso/Miro-FeNC, which can effectively anchor Fe species. The Fe 2p spectrum exhibits two pairs of double peaks, which correspond to Fe2+ (724.7 eV and 710.9 eV) and Fe3+ (728.1 eV and 714.9 eV), along with two satellite peaks [Supplementary Figure 10][44,45]. No peaks associated with Fe0 or other Fe compounds are observed, consistent with the XRD and TEM results. The chemical states of C, N, and Fe in Fe-NC are similar to those in Meso/Micro-FeNC [Supplementary Figures 11-13]. Notably, although Fe-NC and
The coordination environment and atomic dispersion of Fe species in Meso/Micro-FeNC were further investigated using X-ray absorption near-edge structure (XANES) and extended X-ray absorption fine structure (EXAFS). The near-edge energy adsorption threshold lies between Fe2O3 and iron phthalocyanine (FePc), but closer to FePc [Figure 2E]. This indicates an iron valence state intermediate between +2 and +3, with a stronger tendency toward +2. The XANES spectra of both Meso/Micro-FeNC and FePc exhibit a peak at 7,112 eV, characteristic of the square plane of Fe-N4 coordination. The Fourier transform to the
The ORR performance of Meso/Micro-FeNC was evaluated using a rotating disk electrode (RDE) in
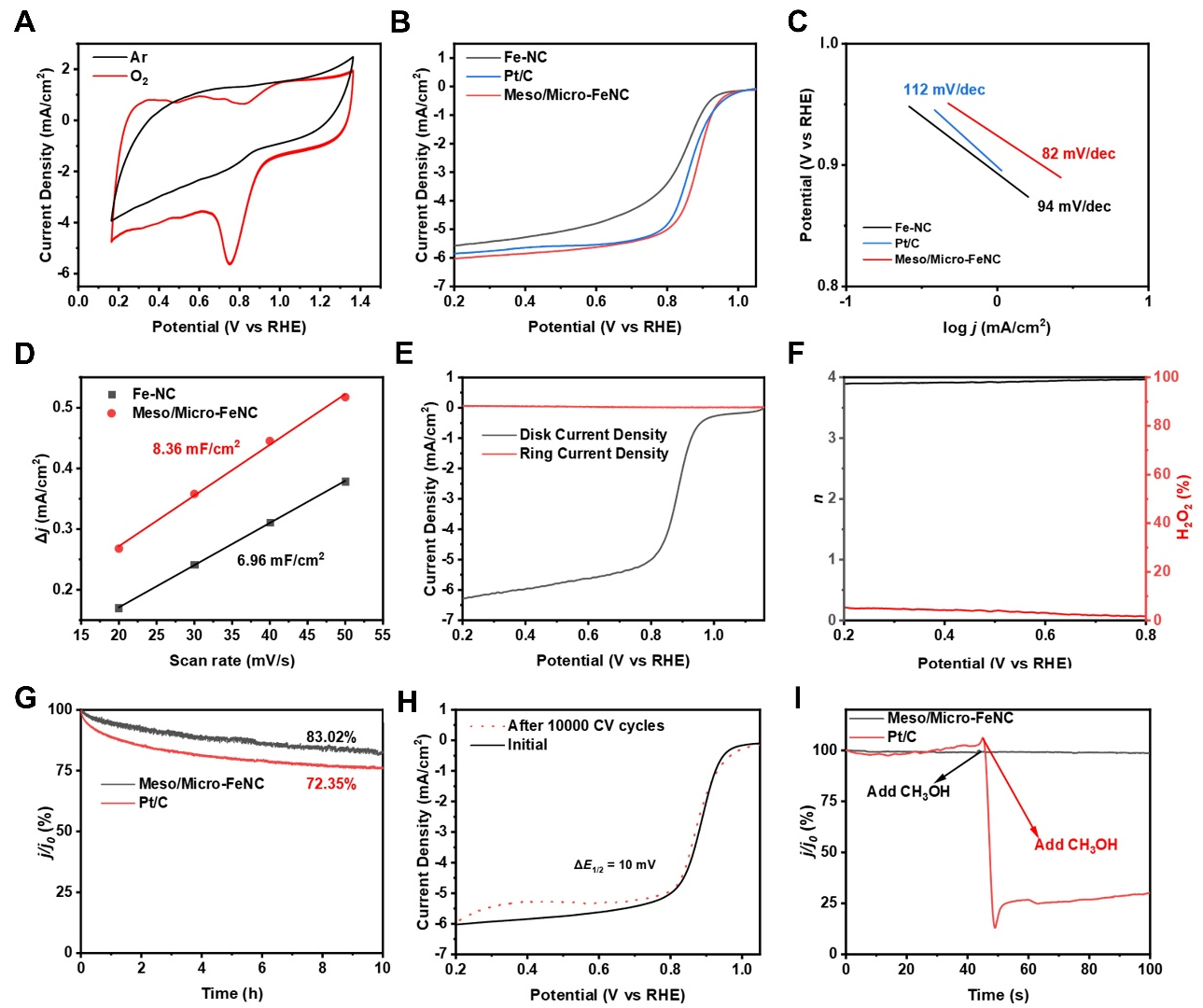
Figure 3. (A) CV curves of Meso/Micro-FeNC under O2/Ar saturation, (B) LSV curves of Meso/Micro-FeNC, Fe-NC and Pt/C catalysts under O2 saturation and 1600 rpm and (C) Tafel slopes of Meso/Micro-FeNC, Fe-NC, and Pt/C catalysts; (D) The Cdl value of Fe-NC and Meso/Micro-FeNC, (E) LSV curves of Meso/Micro-FeNC using RRDE under 1600 rpm and (F) electron transfer number and the yield of H2O2 at different potentials; (G) j-t curves for the catalysts Meso/Micro-FeNC and Pt/C, (H) LSV curves of Meso/Micro-FeNC before and after 10000 cycles of CV test and (I) methanol tolerance experiments on Meso/Micro-FeNC and Pt/C. CV: Cyclic voltammetry; Meso/Micro-FeNC: meso/microporous Fe/N-codoped carbon; LSV: linear scanning voltammetry; RRDE: rotating
To elucidate the ORR reaction pathway of Meso/Micro-FeNC, the number of transferred electrons of
In order to investigate the stability of Meso/Micro-FeNC, the ORR durability tests were conducted
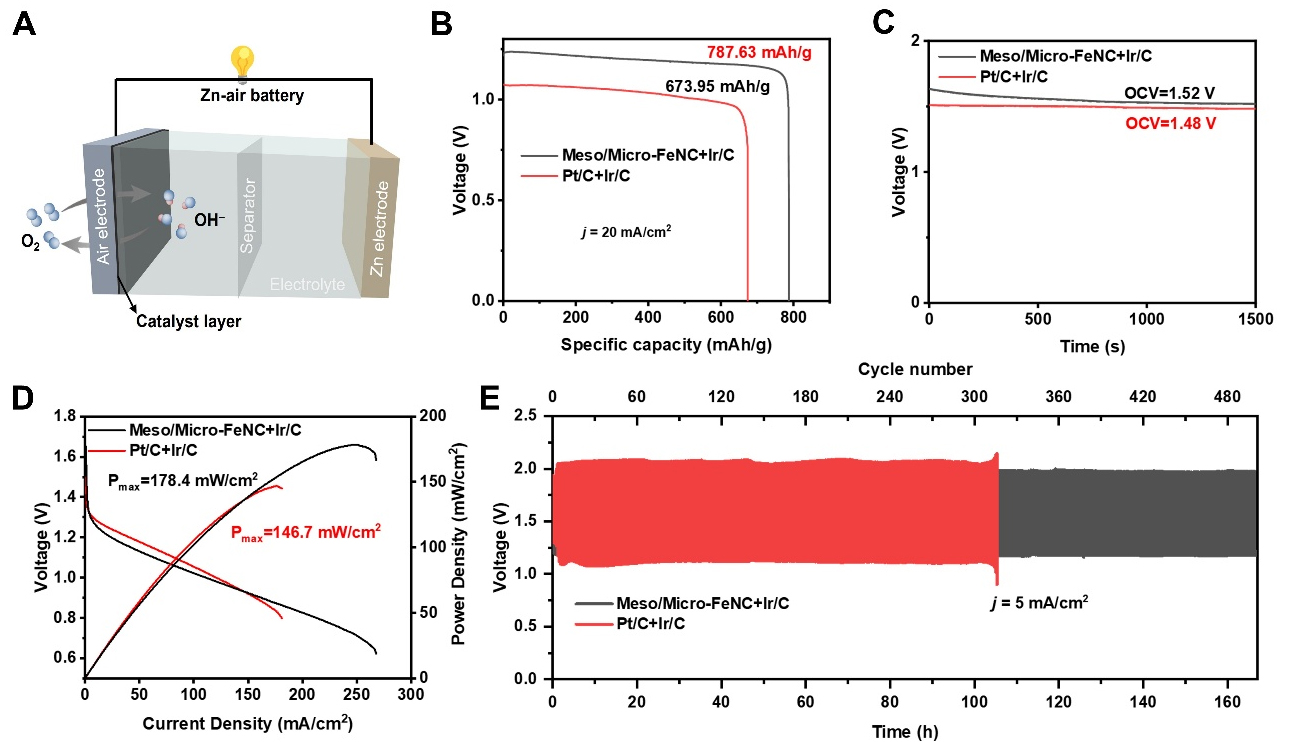
ZABs were assembled using Meso/micro-FeNC catalysts on air electrode and Zn foils as the other electrode, with Pt/C+Ir/C as a benchmark [Figure 4A]. To ensure OER activity, Ir/C was added into air cathode. At a current density of 20 mA cm-2, the discharge specific capacity of Meso/Micro-FeNC+Ir/C was
Figure 4. (A) Electrochemical performances of ZABs assembled with catalysts. Schematic diagram of a ZAB; (B) Discharge specific capacity curves at current density of 20 mA cm-2, (C) open-circuit voltages, (D) discharge polarization curves, and the power densities of Meso/Micro-FeNC+Ir/C and Pt/C+Ir/C; (E) Galvanostatic long-term charge and discharge profiles of Meso/Micro-FeNC+Ir/C and Pt/C+Ir/C. ZABs: Zinc-air batteries; Meso/Micro-FeNC: meso/microporous Fe/N-codoped carbon.
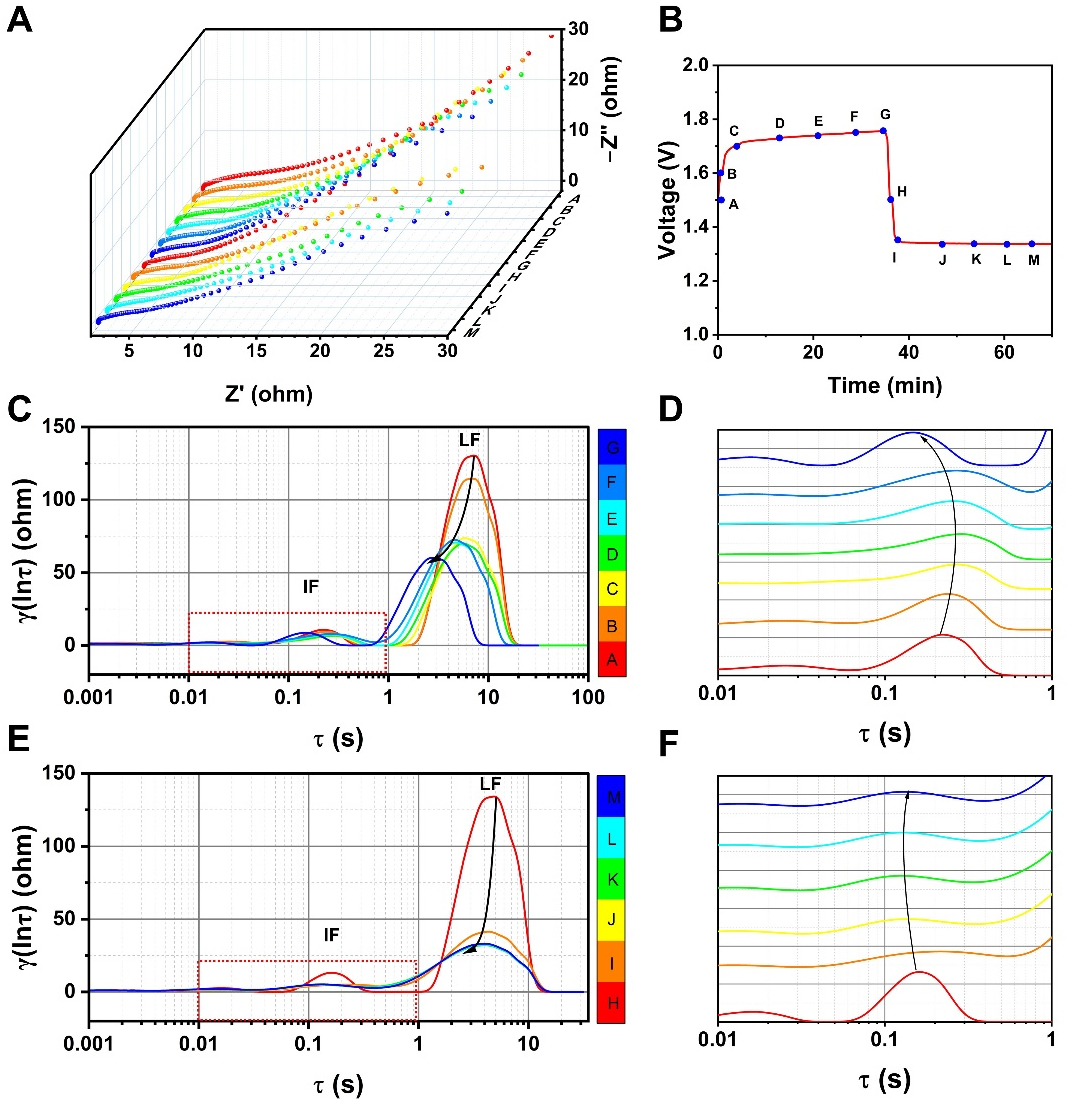
In-situ EIS coupled with distribution of relaxation time (DRT) analysis was applied to investigate the
Figure 5. Electrochemical analysis of Meso/Micro-FeNC and FeNC. (A) In-situ EIS of Meso/Micro-FeNC at different charge-discharge potentials in a ZAB; (B) The corresponding test potentials for in-situ EIS on the initial charge-discharge curves of the ZAB; (C) DRT plot of Meso/Micro-FeNC obtained by deconvolving the in-situ EIS during charge processes; (D) The enlarged view of the IF peak of the charge DRT; (E) DRT plot of Meso/Micro-FeNC obtained by deconvolving the in-situ EIS during discharge processes; (F) The enlarged view of the IF peak of the discharge DRT. Meso/Micro-FeNC: Meso/microporous Fe/N-codoped carbon; ZABs: zinc-air batteries; EIS: electrochemical impedance spectroscopy; DRT: distribution of relaxation time; IF: intermediate-frequency.
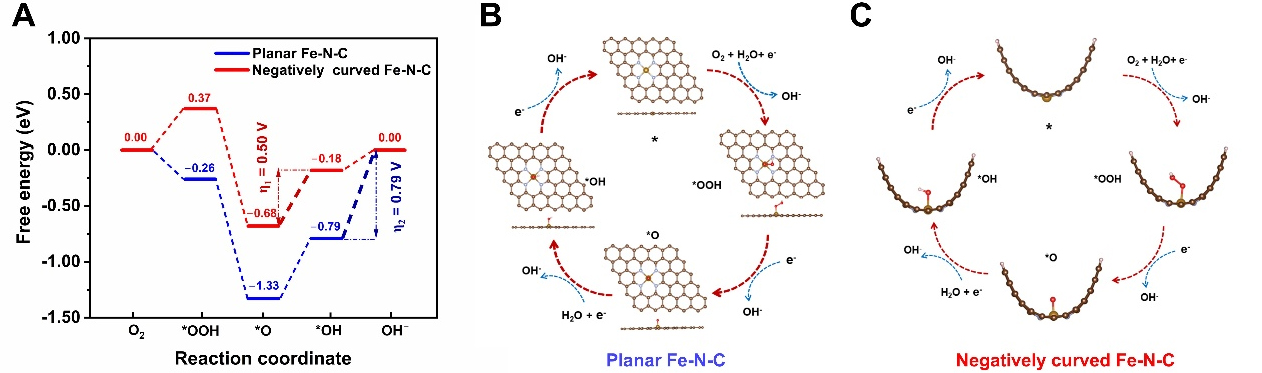
To gain deeper insight into the effect of substrate negative curvature on catalytic activity, we performed DFT calculations of the ORR mechanisms on FeN4 sites supported on both planar and curved graphene. To construct a smooth concave surface, we employed a hydrogen-saturated half-carbon nanotube (CNT) model derived from a (12,0) CNT (radius = 4.88 Å, curvature = 0.20 Å-1), as detailed in the Computational methods section. Figure 6 presents the free energy diagrams for the ORR on planar and curved catalysts at a potential of U = 0.46 V, corresponding to the equilibrium potential at pH = 13. For the negatively curved
CONCLUSIONS
In conclusion, we prepared a single-atom Fe-NC hollow nano-leaf ORR catalyst with microporous and mesoporous structures (Meso/Micro-FeNC) using nano-emulsion-induced self-assembly. Experimental results demonstrate that the structural evolution of the surface coating into hierarchical pores is crucial for improving mass transfer and providing more accessible active sites and. Meso/Micro-FeNC exhibits a high ECSA of 522.5 m2 g-1 and a high density of Fe-N active sites. Benefiting from these advantages, Meso/Micro-FeNC demonstrates exceptional ORR performance, including an E1/2 of
DECLARATIONS
Authors’ contributions
Data curation, formal analysis, investigation, methodology, software, validation, writing - original draft: Song, L.; Liu, Y.
Investigation, data curation, software: Wang, J. J.; Wu, R.
Correcting and conceptualization, formal analysis and supervision: Zhang, H.; Dang, J. S.; Zhang, W.; Cao, R.
Visualization, formal analysis, writing-review & editing, conceptualization, funding acquisition: Zheng, H.
Availability of data and materials
Morphology and structure for the material characterizations and the electrochemical performance of materials for exploration and comparison are presented in the Supplementary Materials. Further data are available from the corresponding author upon reasonable request.
Financial support and sponsorship
We gratefully acknowledge the financial support from National Natural Science Foundation of China (Nos. 22375120 and 21975148), Natural Science Basic Research Program of Shaanxi (No. 2022JC-05), Technology and Innovation Seed Fund (Project CXPY2022092), 111 Project (No. B14041), and International Joint Research Center on Advanced Characterizations of Xi’an City, China.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
Supplementary Materials
REFERENCES
1. Zhu, Z.; Jiang, T.; Ali, M.; et al. Rechargeable batteries for grid scale energy storage. Chem. Rev. 2022, 122, 16610-751.
2. Zhao, Y.; Saseendran, D. P. A.; Huang, C.; et al. Oxygen evolution/reduction reaction catalysts: from in situ monitoring and reaction mechanisms to rational design. Chem. Rev. 2023, 123, 6257-358.
3. Gao, X.; Chen, Y.; Wang, Y.; et al. Next-generation green hydrogen: progress and perspective from electricity, catalyst to electrolyte in electrocatalytic water splitting. Nano-Micro. Lett. 2024, 16, 237.
4. Zhang, H.; Yu, Y.; Yang, D.; et al. Multifunctional quasi-homogeneous catalysts as a new catalytic strategy to boost the performance of Li-O2 batteries. Adv. Mater. 2025, 37, 2413948.
5. Yang, B.; Liu, W.; Gu, T.; Wu, Z. Material design and catalyst-membrane electrode interface engineering for high-performance rechargeable zinc-air batteries. Energy. Storage. Mater. 2025, 74, 103985.
6. Chen, Y.; Wang, G.; Li, J.; et al. Recent advances in bifunctional carbon-based single-atom electrocatalysts for rechargeable zinc-air batteries. Green. Chem. 2025, 27, 293-324.
7. Wang, Q.; Kaushik, S.; Xiao, X.; Xu, Q. Sustainable zinc-air battery chemistry: advances, challenges and prospects. Chem. Soc. Rev. 2023, 52, 6139-90.
8. Lv, X. W.; Wang, Z.; Lai, Z.; et al. Rechargeable zinc-air batteries: advances, challenges, and prospects. Small 2024, 20, e2306396.
9. Yang, Z.; Chen, Y.; Zhang, S.; Zhang, J. Identification and understanding of active sites of non-noble iron-nitrogen-carbon catalysts for oxygen reduction electrocatalysis. Adv. Funct. Mater. 2023, 33, 2215185.
10. Liu, L.; Rao, X.; Zhang, S.; Zhang, J. Insight into synergy for oxygen reduction electrocatalysis of iron-nitrogen-carbon. Chem 2024, 10, 1994-2030.
11. Zhang, Z.; Liu, J.; Curcio, A.; et al. Atomically dispersed materials for rechargeable batteries. Nano. Energy. 2020, 76, 105085.
12. Li, L.; Tang, X.; Wu, B.; Huang, B.; Yuan, K.; Chen, Y. Advanced architectures of air electrodes in zinc-air batteries and hydrogen fuel cells. Adv. Mater. 2024, 36, 2308326.
13. Lei, H.; Ma, L.; Wan, Q.; et al. Porous carbon nanofibers confined NiFe alloy nanoparticles as efficient bifunctional electrocatalysts for Zn-air batteries. Nano. Energy. 2022, 104, 107941.
14. Chen, D.; Yu, R.; Yu, K.; et al. Bicontinuous RuO2 nanoreactors for acidic water oxidation. Nat. Commun. 2024, 15, 3928.
15. Yin, S.; Chen, L.; Yang, J.; et al. A Fe-NC electrocatalyst boosted by trace bromide ions with high performance in proton exchange membrane fuel cells. Nat. Commun. 2024, 15, 7489.
16. Zhan, Y.; Zhao, T.; Wu, X.; et al. Strengthening the oxygen reduction stability and activity of single iron active sites via a simultaneously electronic regulation and structure design strategy. Appl. Catal. B. Environ. Energy. 2024, 357, 124254.
17. Liu, J.; Gong, Z.; Allen, C.; et al. Edge-hosted Fe-N3 sites on a multiscale porous carbon framework combining high intrinsic activity with efficient mass transport for oxygen reduction. Chem. Catalysis. 2021, 1, 1291-307.
18. Yang, C.; Chen, J.; Yan, L.; Gao, Y.; Ning, J.; Hu, Y. Customizing oxygen electrocatalytic microenvironment with S, N codoped carbon nanofibers confining carbon nanocapsules and Co9S8 nanoparticles for rechargeable Zn-air batteries. Appl. Catal. B. Environ. Energy. 2024, 352, 124060.
19. Chen, K.; Liang, Y.; Pan, D.; et al. Mass transportation facilitated porous Fe/Co dual-site catalytic cathodes for ultrahigh-power-density Al-air fuel cells. Adv. Energy. Mater. 2025, 15, 2404140.
20. Zhai, W.; Li, J.; Tian, Y.; et al. Consolidating the oxygen reduction with sub-polarized graphitic Fe-N4 atomic sites for an efficient flexible zinc-air battery. Nano. Lett. 2024, 24, 14632-40.
21. Zhao, Y.; Zhu, L.; Tang, J.; et al. Enhancing electrocatalytic performance via thickness-tuned hollow N-doped mesoporous carbon with embedded Co nanoparticles for oxygen reduction reaction. ACS. Nano. 2024, 18, 373-82.
22. Li, J. K.; Zhao, H.; Zhang, Y.; et al. In situ electron tomography insights into the curvature effect of a concave surface on Fe single atoms for durable oxygen reaction. Adv. Sci. 2025, 12, 2412387.
23. Zhang, P.; Chen, H. C.; Zhu, H.; et al. Inter-site structural heterogeneity induction of single atom Fe catalysts for robust oxygen reduction. Nat. Commun. 2024, 15, 2062.
24. Zhai, X.; Qu, J.; Wang, J.; et al. Diffusion-driven fabrication of yolk-shell structured K-birnessite@mesoporous carbon nanospheres with rich oxygen vacancies for high-energy and high-power zinc-ion batteries. Energy. Storage. Mater. 2021, 42, 753-63.
25. Song, X.; Zhang, J.; Feng, X.; Qi, Y.; Cui, J.; Xiong, Y. CoFe2O4/CoFe loaded 3D ordered hierarchical porous N-doped carbon for efficient oxygen reduction in Zn-air battery and hydrogen evolution. J. Energy. Chem. 2025, 106, 220-30.
26. Zhang, W.; Li, H.; Feng, D.; et al. MOF-derived 1D/3D N-doped porous carbon for spatially confined electrochemical CO2 reduction to adjustable syngas. Carbon. Energy. 2024, 6, e461.
27. Wang, Y.; Sun, T.; Mostaghimi, A. H. B.; et al. Two-dimensional metal-organic frameworks with unique oriented layers for oxygen reduction reaction: tailoring the activity through exposed crystal facets. CCS. Chem. 2022, 4, 1633-42.
28. Chen, R.; Yao, J.; Gu, Q.; et al. A two-dimensional zeolitic imidazolate framework with a cushion-shaped cavity for CO2 adsorption. Chem. Commun. 2013, 49, 9500-2.
29. Wang, T.; Kou, Z.; Mu, S.; et al. 2D dual-metal zeolitic-imidazolate-framework-(ZIF)-derived bifunctional air electrodes with ultrahigh electrochemical properties for rechargeable zinc-air batteries. Adv. Funct. Mater. 2018, 28, 1705048.
30. Shen, J.; Aljarb, A.; Cai, Y.; et al. Engineering grain boundaries in monolayer molybdenum disulfide for efficient water-ion separation. Science 2025, 387, 776-82.
31. Qin, J.; Yang, Z.; Xing, F.; Zhang, L.; Zhang, H.; Wu, Z. Two-Dimensional mesoporous materials for energy storage and conversion: current status, chemical synthesis and challenging perspectives. Electrochem. Energy. Rev. 2023, 6, 9.
32. He, J.; Wang, W.; Yan, J.; et al. Stabilizing electron transport of 2D materials. Adv. Mater. 2025, 37, 2411941.
33. Zhao, B.; Luo, H.; Liu, J.; et al. S-doped carbonized wood fiber decorated with sulfide heterojunction-embedded S, N-doped carbon microleaf arrays for efficient high-current-density oxygen evolution. Chin. Chem. Lett. 2025, 36, 109919.
34. Fan, Y.; Wang, W.; Chen, Y.; et al. Cobalt-containing ZIF-derived catalysts for Zn-air batteries. Mater. Chem. Front. 2024, 8, 2394-419.
35. Guo, X.; Zhang, H.; Yao, Y.; et al. Stabilizing atomic Co on 2D ordered mesoporous carbon sandwiched MXene for peroxymonosulfate activation: Enhanced performance and electron-transfer mechanism. Appl. Catal. B. Environ. Energy. 2024, 358, 124432.
36. Ma, M.; Pei, Z.; Peng, Y.; et al. Single Zn atoms anchored in mesoporous N-doped carbon rods derived from metal-organic frameworks for enhanced electrocatalytic oxygen reduction reaction. J. Mater. Chem. A. 2025, 13, 7529-38.
37. Huang, Z.; Ma, D.; Nian, P.; et al. Coordinating interface polymerization with micelle mediated assembly towards two-dimensional mesoporous carbon/CoNi for advanced lithium-sulfur battery. Small 2023, 19, 2207411.
38. Dai, X.; Zhao, Z. ZIF-L-derived FeN-hcC catalysts with curved carbon surfaces for effective oxygen reduction reaction over the entire pH range. New. J. Chem. 2024, 48, 18719-27.
39. Xu, Y.; Hou, W.; Huang, K.; et al. Engineering built-in electric field microenvironment of CQDs/g-C3N4 heterojunction for efficient photocatalytic CO2 reduction. Adv. Sci. 2024, 11, 2403607.
40. Zhang, S.; Wu, J.; Zheng, M.; et al. Fe/Cu diatomic catalysts for electrochemical nitrate reduction to ammonia. Nat. Commun. 2023, 14, 3634.
41. He, J.; Li, N.; Li, Z.; et al. Strategic defect engineering of metal-organic frameworks for optimizing the fabrication of single-atom catalysts. Adv. Funct. Mater. 2021, 31, 2103597.
42. Li, W.; Chen, L.; Qiu, M.; et al. Highly efficient epoxidation of propylene with in situ -generated H2O2 over a hierarchical TS-1 zeolite-supported non-noble nickel catalyst. ACS. Catal. 2023, 13, 10487-99.
43. Fan, X. Z.; Du, X.; Pang, Q. Q.; Zhang, S.; Liu, Z. Y.; Yue, X. Z. In situ construction of bifunctional N-doped carbon-anchored Co nanoparticles for OER and ORR. ACS. Appl. Mater. Interfaces. 2022, 14, 8549-56.
44. Chen, Z.; Xu, W.; Wang, W.; et al. Bamboo-like carbon nanotube-encapsulated Fe2C nanoparticles activate confined Fe2O3 nanoclusters via d-p-d orbital coupling for alkaline oxygen evolution reaction. Small 2025, 21, 2409325.
45. Tang, X.; Wei, Y.; Zhai, W.; et al. Carbon nanocage with maximum utilization of atomically dispersed iron as efficient oxygen electroreduction nanoreactor. Adv. Mater. 2023, 35, 2208942.
46. Lyu, L.; Hu, X.; Lee, S.; et al. Oxygen reduction kinetics of Fe-N-C single atom catalysts boosted by pyridinic N vacancy for temperature-adaptive Zn-air batteries. J. Am. Chem. Soc. 2024, 146, 4803-13.
47. Zhu, Y.; Jiang, Y.; Li, H.; et al. Tip-like Fe-N4 sites induced surface microenvironments regulation boosts the oxygen reduction reaction. Angew. Chem. Int. Ed. 2024, 63, e202319370.
48. Wang, Y.; Yang, T.; Fan, X.; et al. Anchoring Fe species on the highly curved surface of S and N Co-doped carbonaceous nanosprings for oxygen electrocatalysis and a flexible zinc-air battery. Angew. Chem. Int. Ed. 2024, 63, e202313034.
49. Sang, W.; Chaemchuen, S.; Zhang, L.; et al. Solid-state stepwise temperature-programmable synthesis of bioinspired Fe-N-C oxygen reduction electrocatalyst featuring Fe-N5 configuration. Nano. Res. 2025, 18, 94907245.
50. Bae, G.; Kwon, H. C.; Han, M. H.; Oh, H.; Jaouen, F.; Choi, C. H. Single-site-level deciphering of the complexity of electrochemical oxygen reduction on Fe-N-C catalysts. ACS. Catal. 2024, 14, 8184-92.
51. Jia, C.; Zhao, Y.; Song, S.; et al. Highly ordered hierarchical porous single-atom Fe catalyst with promoted mass transfer for efficient electroreduction of CO2. Adv. Energy. Mater. 2023, 13, 2302007.
52. Liu, S.; Meyer, Q.; Jia, C.; et al.
53. Liu, Y.; Li, J.; Lv, Z.; et al. Efficient proton-exchange membrane fuel cell performance of atomic Fe sites via p-d hybridization with Al dopants. J. Am. Chem. Soc. 2024, 146, 12636-44.
54. Wang, X.; Zhang, J.; Wang, P.; et al. Terbium-induced cobalt valence-band narrowing boosts electrocatalytic oxygen reduction. Energy. Environ. Sci. 2023, 16, 5500-12.
55. Li, J.; Zhu, Y.; Chen, W.; et al. Breathing-mimicking electrocatalysis for oxygen evolution and reduction. Joule 2019, 3, 557-69.
56. Jiang, S.; Xiang, Q.; Xie, Z.; et al. Influence of the Pt/ionomer/water interface on the oxygen reduction reaction: insights into the micro-three-phase interface. Chem. Sci. 2024, 15, 19290-8.
57. Yan, L.; Xie, B.; Yang, C.; et al. Engineering self-supported hydrophobic-aerophilic air cathode with CoS/Fe3S4 nanoparticles embedded in S, N Co-doped carbon plate arrays for long-life rechargeable Zn-air batteries. Adv. Energy. Mater. 2023, 13, 2204245.
58. Zhao, Y.; Shi, Z.; Li, F.; et al. Deciphering mesopore-augmented CO2 electroreduction over atomically dispersed Fe-N-doped carbon catalysts. ACS. Catal. 2024, 14, 3926-32.
59. Wu, J.; Zhu, Y.; Cai, A.; Fan, X.; Peng, W.; Li, Y. Structural engineering of Fe single-atom oxygen reduction catalyst with high site density and improved mass transfer. J. Energy. Chem. 2024, 98, 634-44.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Data & Comments
Data

















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.