Photo aging of polyester microfiber in freshwater and seawater environments: kinetics, mechanisms, and influencing factors
Abstract
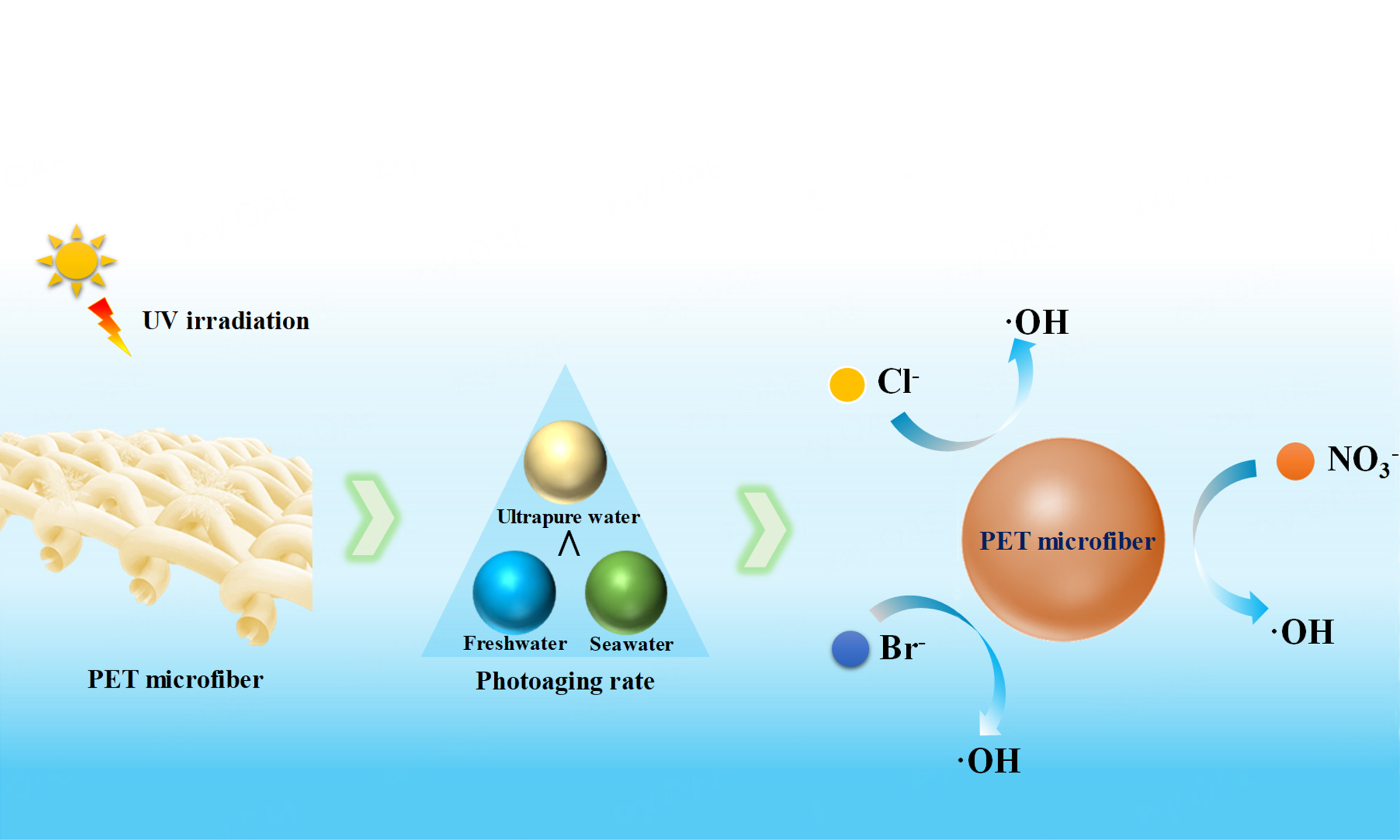
Microfibers, particularly polyethylene terephthalate (PET, commonly known as polyester), are the predominant form of microplastic pollution in aquatic environments. However, the process by which PET microfibers form in these environments remains unclear. To investigate this, we exposed PET microfibers to both freshwater and seawater environments and subjected them to ultraviolet irradiation for 12 days. According to atomic force microscopy, X-ray photoelectron spectroscopy, differential scanning calorimetry, and gel permeation chromatography analyses, PET microfibers exhibited diverse photoaging behavior in freshwater and seawater environments, with the photoaging rate in seawater higher than in freshwater and ultrapure water. Photochemically active ions, including Cl-, Br-, and NO3-, are identified as the dominant factors controlling the aging rate of PET microfibers, particularly NO3-. Mechanistic insights suggest that this effect is due to the higher steady-state concentration of •OH produced in solutions containing these ions (6.04 × 10-15 M for Cl-, 4.93 × 10-15 M for Br-, and 8.00 × 10-15 M for NO3-) compared to pure water (3.72 × 10-15 M), which further accelerates PET photoaging. These findings provide an in-depth understanding of the formation and fate of PET microfibers in freshwater and seawater environments.
Keywords
INTRODUCTION
Microfibers are linear synthetic fiber particles with a length-to-diameter ratio greater than 3, typically ranging from 100 μm to 5 mm[1]. As emerging contaminants, microfibers have been widely detected in global aquatic environments. In seawater, reported abundances include 4,900 n/m3 in the Yellow Sea[2], 127 n/m3 in the South China Sea[3], 1,781 n/m3 in the Gulf of Thailand[4], 1.15 n/m3 in South Atlantic[5], and 0.51 n/m3 in the central Pacific[6]. In freshwater, concentrations range from 393.1-17,403.8 n/m3 in the Yangtze River, China[7], 8.2 × 104 n/m3 in the Awano River, Japan[8], 1.4 × 106 n/m3 in the Ganges River, India[9], and
After being released into freshwater and marine environments, microfibers can undergo photochemical transformation during long-term residence. Continuous photoaging of microplastics can produce microcracks, depressions, or protrusions on fiber surfaces, increasing surface roughness. Plastic photoaging in water occurs when sunlight irradiation, particularly ultraviolet (UV) light, excites unsaturated bonds in the polymer. This triggers a series of chemical reactions, causing breakage or recombination of molecular chains and resulting in changes to the material’s physical and chemical properties. Over time, these microcracks may expand and deepen, further compromising the structural integrity of the material, resulting in fragmentation and size reduction of the aged plastic fibers. In the study by Sørensen et al. PET fibers developed micrometer-sized pores after 56 days of UV exposure and eventually broke into smaller particles, with the median fiber length decreasing from 3,115 μm before exposure (day 0) to 257 μm after 56 days[17]. This is due to the continuous UV irradiation, which inevitably breaks the PET polymer chain, interfering with the molecular structure and crystal arrangement of plastics, which tends to embrittle and break into small plastic fragments under mechanical forces induced by water flow. Such surface roughening increases the specific surface area and available adsorption sites of microplastics, thereby affecting their interactions with other substances in the environment. For example, after UV irradiation, PET microplastics exhibited a significant enhancement in Cu2+ adsorption capacity, reaching twice that of pristine microplastics[18]. There are also notable differences in the adsorption behavior (including adsorption capacity and interaction mechanisms) of aged microplastics toward hydrophilic organic pollutants such as ciprofloxacin - aged polystyrene (PS) and poly(vinyl chloride) showed 123.3% and 20.4% higher adsorption capacities compared to their pristine counterparts, respectively[19]. Sharma et al. (2022) further reported that naturally aged fiber microplastics had a heavy metal adsorption capacity of 30.8 mg/g, which is about 35% higher than that of original synthetic fibers[20]. These indicate that microfibers distributed in current natural water bodies are of different aging degrees, with aged microfibers may have higher ecological risks than that of pristine ones. Exploring the aging behavior and mechanism of microfiber in seawater and freshwater systems can help evaluate the ecological risks of microfiber in natural water bodies.
Furthermore, complex aqueous chemical conditions - such as water temperature, pH, inorganic ions, and natural organic matter - can also influence the oxidative degradation of microplastics in fibers[21]. For instance, Zhu et al. (2022) investigated the photoaging process of PS microplastics (PS-MP) in the presence of common inorganic anions (NO3-, Cl-, and Br-), and discovered that NO3-, Cl-, and Br- ions promoted the indirect photoaging of PSMP by modulating the concentration of hydroxyl radicals (•OH)[22]. This indicates that the type and concentration of inorganic ions can affect the photodegradation of microplastics in water[22]. Similarly, Deng et al. (2024) examined the photoaging kinetics and mechanisms of PS in the presence of common inorganic cations [Fe(III), NH4+, Ca2+, and Na+][23]. The results indicated that Fe(III) inhibited the photoaging of PSMP by reducing light transmittance via photon shielding, and NH4+ suppressed photoaging by consuming hydroxyl radicals. In both cases, the inhibitory effect increased with higher ion concentrations. In contrast, Ca2+ and Na+ had no significant impact on the photoaging of PSMP[23]. Therefore, the photoaging of microfiber in aquatic environments is a complex process influenced by a variety of intrinsic and extrinsic factors. Thus, elucidating the aging and formation mechanisms of microfibers in natural water bodies requires comprehensive consideration of the various aqueous chemical conditions. Understanding these influencing mechanisms is crucial for assessing the stability and risks of microfibers in aquatic ecosystems.
Based on the above considerations, this work systematically investigates the photoaging of PET microfibers in freshwater and seawater environments. The objectives are to: (1) examine the aging behavior and rate of PET microfibers in freshwater and seawater; (2) identify the dominant factors controlling their aging rate in both environments; and (3) elucidate the mechanisms governing PET microfiber aging. The findings provide valuable insights for assessing the fate and ecological risks of PET microfibers in natural waters.
MATERIALS AND METHODS
Materials
PET microfiber (5 mm in length) was obtained from Fujix (Shanghai) Sewing Thread Co. Ltd. China. Seawater was sampled on October 10, 2022, from the Weihai area, Shandong Province, China (122°1′10″E, 37°31′7″N), with pH 6.45, salinity 22.4 g/L, electrical conductivity 12.9 mS/cm, and total organic carbon 3.51 mg/L. Freshwater was collected in Beijing, China, on February 10, 2023, with pH 7.19, salinity 0.109 g/L, conductivity 0.229 mS/cm, and total organic carbon 9.96 mg/L. All natural water samples were stored at 4 °C before use. Purified water was prepared using a Milli-Q IQ 7000 ultrapure water purification system (ZIQ7000T0C, Sigma-Aldrich, Shanghai, China). Chemical reagents, including sodium chloride (NaCl,
Photoaging experiment of PET microfiber in freshwater and seawater
Photoaging of PET microfiber in freshwater and seawater was conducted using a photochemical reaction apparatus (CEL-LAB500, Beijing Zhongjiao Jinyuan Technology Co. Ltd.) equipped with a 500 W mercury lamp. Specifically, 100 mg of PET microfibers were mixed with 15 mL of natural water samples (6.66 g/L) and irradiated under UV light for 12 days. The reaction temperature was maintained at 25 °C using a circulating condensate water system. Aged PET microfibers were collected after 2, 4, 6, 8, 10, and 12 days of UV exposure by filtration through a 3 μm stainless steel membrane, dried at 50 °C for 48 h, and stored at
Characterization for the pristine and aged PET microfiber
The surface morphology and roughness distribution of aged PET microfibers after UV aging in water were characterized using atomic force microscopy (AFM, Bruker Dimension Icon, Germany). AFM images were analyzed using NanoScope software to determine the average roughness (Ra). X-ray photoelectron spectroscopy (XPS, ESCALAB 250Xi, Thermo, USA) was used to analyze the elemental distribution (carbon and oxygen) and the formation of functional groups (carbonyl and hydroxyl) on the PET microfiber surface. Differential scanning calorimetry (DSC, Discovery DSC2500, Waters, USA) was used to measure the melting point (Tm), crystallization temperature (Tc), and enthalpy changes of both pristine and aged PET fibers. The DSC procedure was as follows: under a nitrogen atmosphere, the sample was equilibrated at 30 °C, then heated to 300 °C at 10 °C/min, held at 300 °C for 5 min, and finally cooled to 30 °C at 10 °C/min. Gel permeation chromatography (GPC, PL-GPC220, Agilent, USA) was used to determine the molecular weight distribution and changes in polyester fibers before and after photoreaction. The column used was an Agilent PLgel MIXED B (7.5 mm × 300 mm), with cresol as the eluent, a flow rate of 0.5 mL/min, and an injection volume of 200 μL. The hydrophobicity of pristine and aged PET microfibers was assessed using a water contact angle goniometer (LSA100, Lauda Scientific, China). During testing, 2.0 μL of ultrapure water was dispensed onto the sample surface, and images were captured within 1 s after droplet release.
Qualitative and quantitative identification of oxidative free radicals (hydroxyl radicals) during PET microfiber aging in water
In this study, electron paramagnetic resonance (EPR, EMXplus-6/1, Bruker, Germany) was employed to qualitatively identify hydroxyl radicals generated during photoaging of PET microfibers in water, using
Additionally, NB was used to quantitatively determine the •OH formed by PET fibers under UV irradiation, with the second-order kinetic constant for the reaction of NB with •OH being 3.90 × 109 M-1·S-1[24]. In the •OH trapping system, 100 mg of PET fibers was added to a mixture containing 2 mM NB, with a total volume of 20 mL for the aqueous sample. After 0, 1, 2, 3, 4, and 5 h of UV irradiation, 0.5 mL samples were withdrawn using a syringe and filtered through a 0.45 μm glass fiber filter. The concentration of NB was determined using high-performance liquid chromatography (HPLC). The UV detector wavelength, column type, mobile phase, flow rate, column temperature, and injection volume were set as follows: 263 nm; C18 reverse-phase column (5 μm × 250 mm × 4 mm); acetonitrile/water (60:40, V/V); 0.5 mL/min; 30 °C; and 10 μL, respectively.
Quality assurance/quality control (QA/QC)
To prevent potential microplastic contamination from the laboratory environment and supplies during aging and characterization experiments, the following measures were implemented: (1) Experiments were conducted in rooms with fume hoods, keeping the hoods open to ensure timely air renewal and purification; (2) All personnel wore cotton lab coats instead of polyester ones; (3) All pristine and aged microplastic particles were stored in glass containers, pre-cleaned with anhydrous ethanol and ultrapure water.
Statistical analysis
Note that statistical analyses, including the •OH formation capacity of PET microfiber in Cl-, Br-, and NO3--containing aqueous solutions, were performed using Origin 18.0 software. Experiments for •OH quantification were conducted in triplicate, and results are reported as mean ± standard deviation.
RESULTS AND DISCUSSION
Characterization of aged PET microfiber in freshwater and seawater environments
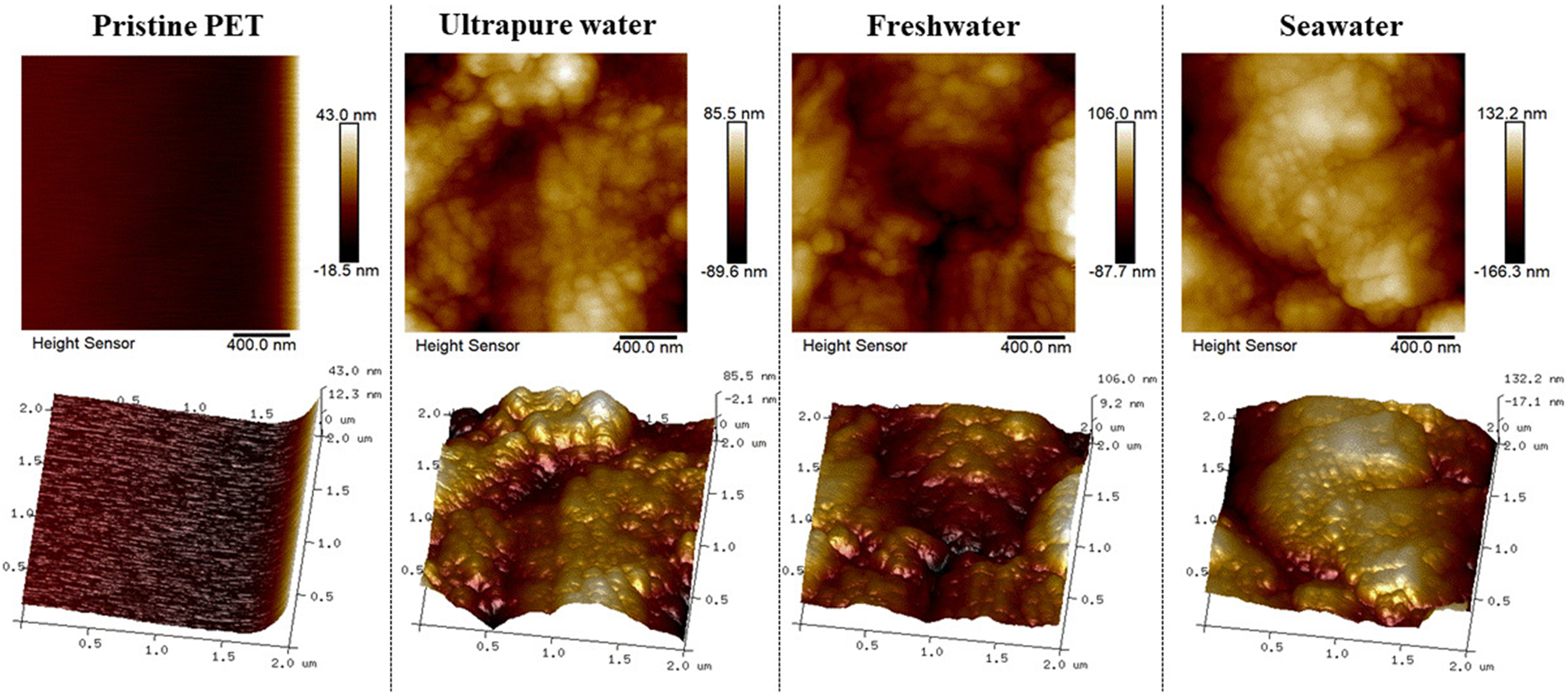
As shown in Figure 1, AFM analysis revealed that after 12 days of photoaging in water, the surface morphology of PET microfibers changed markedly from smooth to rough. NanoScope software was used to calculate the average roughness, which increased from 4.96 nm for pristine PET fibers to 19.4, 20.5, and
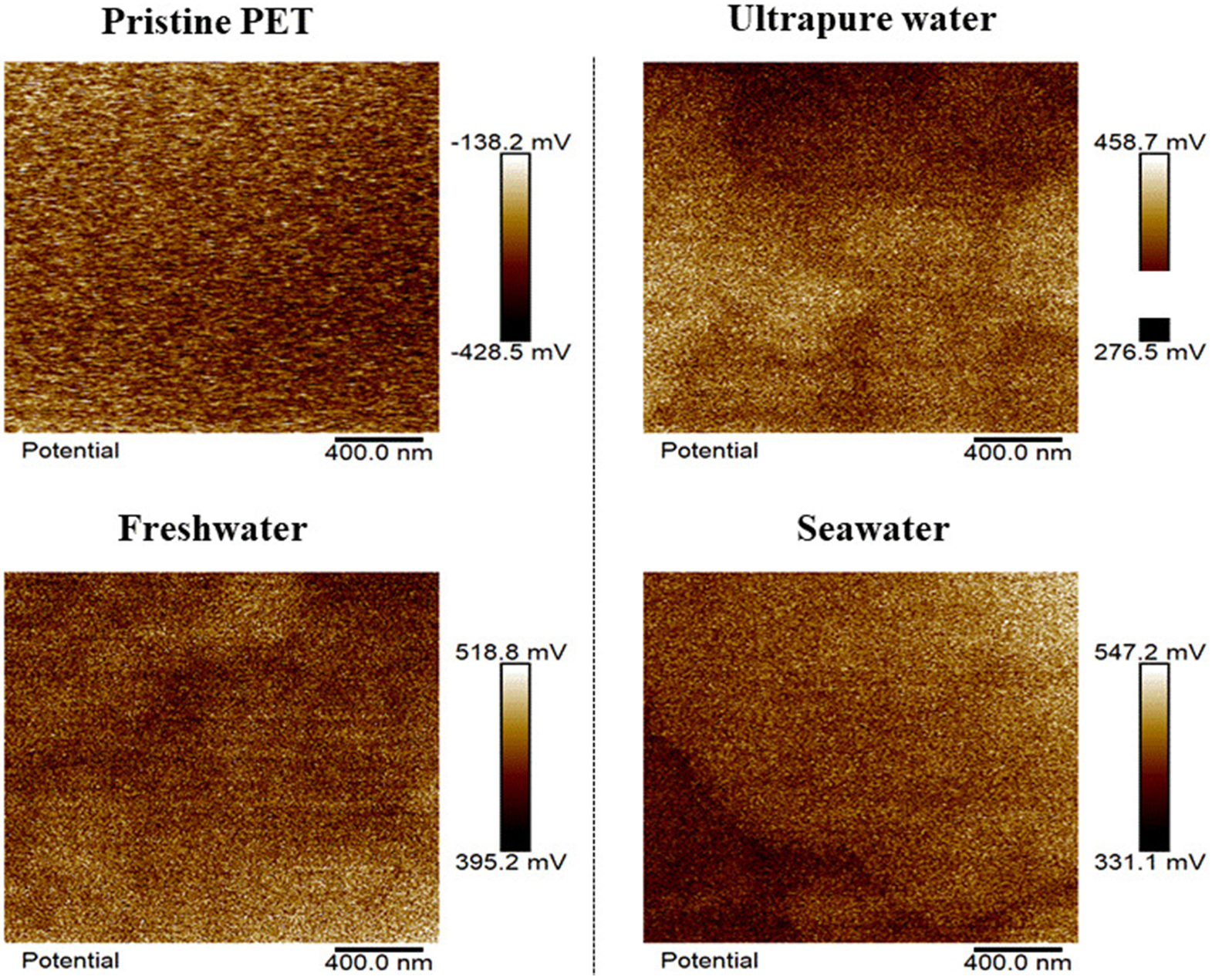
Figure 1. AFM spectra for PET after 12 d of photoaging in ultrapure, freshwater, and seawater. AFM: Atomic force microscopy; PET: polyethylene terephthalate.
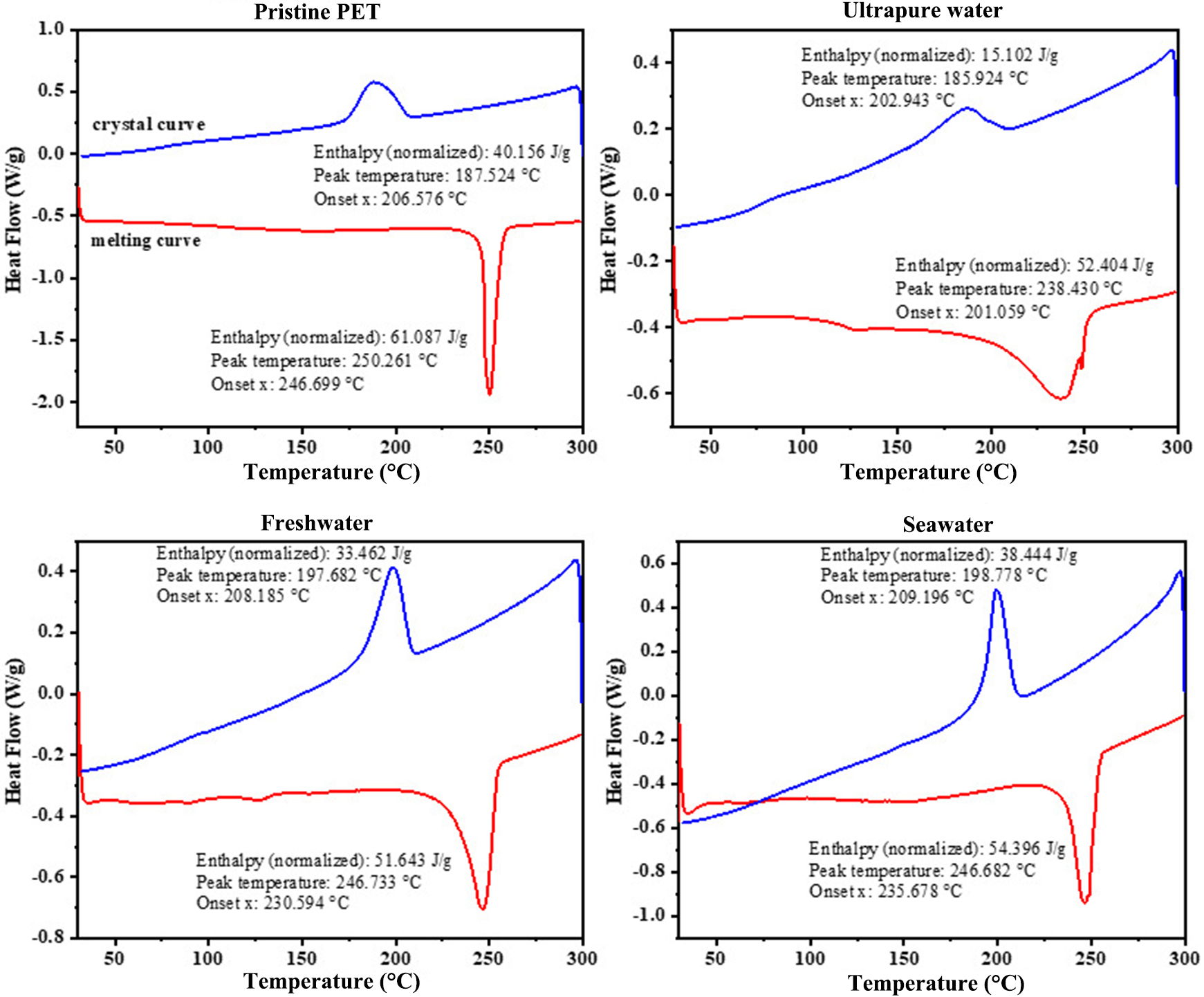
Subsequently, we investigate changes in the physicochemical properties (i.e. melting temperature and crystallinity) of PET fibers across different aqueous environments. The melting and crystallization curves of pristine PET fibers and those photo-aged for 12 days in different aqueous environments were obtained by DSC [Figure 2 and Supplementary Table 1]. The crystallinity of different fibers (Xc) was calculated based on the enthalpy changes of melting and crystallization, as given in
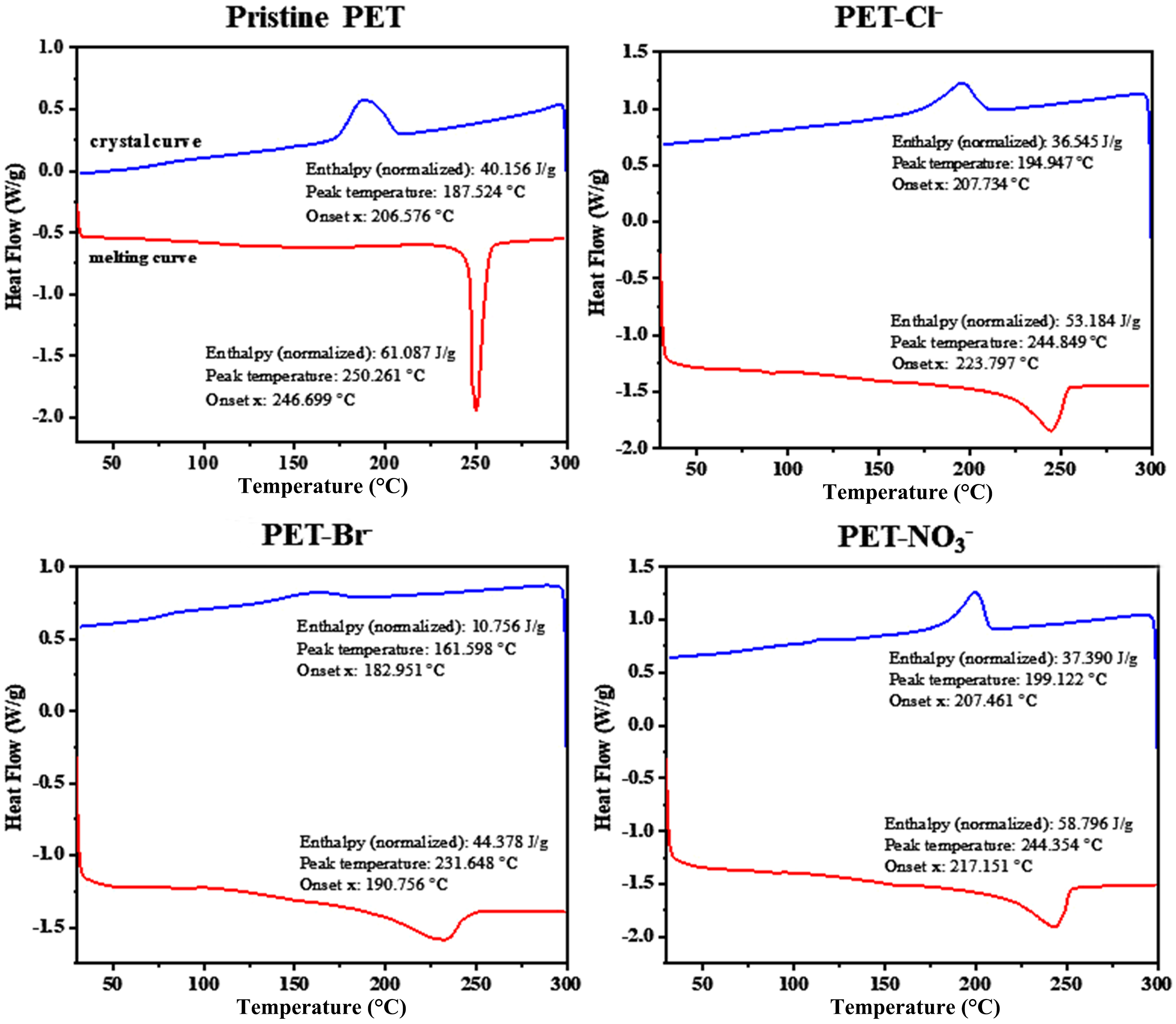
Figure 2. DSC melting and crystal curves of aged PET in different water environments. DSC: Differential scanning calorimetry; PET: polyethylene terephthalate.
where ∆Hm (J/g) is the enthalpy of melting, referring to the heat absorbed when a substance transitions from solid to liquid during heating; ∆Hc is the enthalpy of crystallization, referring to the heat released when a substance transitions from liquid to solid (crystalline state) during cooling. ∆Hm0 is the enthalpy of melting for fully crystalline PET (140 J/g)[26]. Compared to the pristine PET microfiber (Tc = 250.26 °C, Tm =
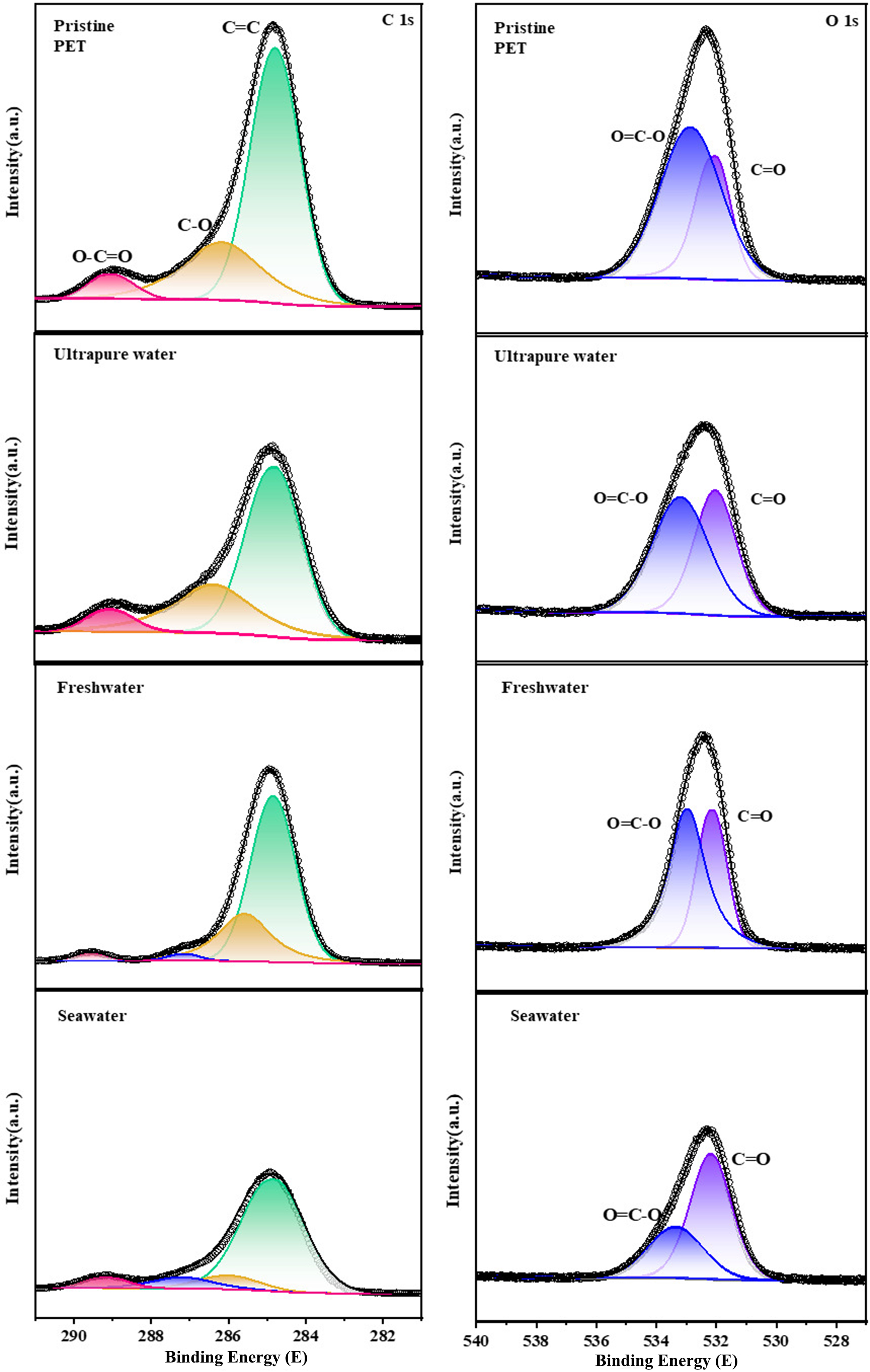
Peak deconvolution and simulation analysis of the C 1s and O 1s spectra of aged PET microfibers were performed using Avantage software, with the results shown in Figure 3. In pristine PET microfiber, the C 1s spectrum exhibits three main peaks: one corresponding to carbon atoms in the ester bond (O=C-O) at
Figure 3. XPS spectra of PET fibers after 12 days of aging in different water environments, including ultrapure water, freshwater, and seawater. XPS: X-ray photoelectron spectroscopy; PET: polyethylene terephthalate.
Kelvin probe force microscopy (KPFM) was used to evaluate changes in the surface charge properties of PET microfibers during photoaging in water [Figure 4]. The maximum surface potential of the pristine PET microfiber was -138.2 mV. After UV irradiation, the maximum surface potential increased to 458.7 mV in ultrapure water, 518.8 mV in freshwater, and 547.2 mV in seawater. Changes in surface charge were also assessed through water contact angle measurements, where a lower contact angle indicates reduced hydrophobicity of PET plastics[32]. Supplementary Figure 1 shows that the contact angle of pristine PET fibers was 108.8 ± 5.5°, which decreased after photoaging by 20.46% in ultrapure water, 28.98% in freshwater, and 35.09% in seawater. These results are consistent with the KPFM analysis and further indicate that the aging rate of PET microfiber is higher in freshwater and seawater than in ultrapure water. Photoaging induces the cleavage of carboxyl and ester groups, generating new oxygen-containing functional groups, such as hydroxyl groups[31]. Mechanistically, photoaging alters the microstructure of the microplastic surface, creating a rougher morphology[33,34]. UV exposure of PET microfibers readily produces oxygen-containing functional groups, including carbonyl and hydroxyl groups[35]. These functional groups enhance interactions between the microfiber surface and water, increasing hydrophilicity. Enhanced hydrophilicity may influence environmental behavior and interactions of microplastics, such as increasing their adsorption capacity for pollutants[36].
Factors controlling the PET fiber photoaging behavior in freshwater and seawater
Preliminary factors controlling the diverse photoaging behavior of PET microfibers in freshwater and seawater were investigated by examining the effects of photochemically active ions, including Cl-, Br-, and NO3- on PET microfiber photoaging. These ions have long been recognized as essential photochemically active constituents with significant impacts on the photodegradation of organic contaminants, including microplastics in water[32,37]. As shown in Figure 5, according to DSC analysis, after 12 days of UV irradiation, the melting temperature (Tm) of PET microfibers decreased from 250.26 to 244.84 °C in 3 mg/L Cl--containing water, 231.64 °C in 3 mg/L Br--containing water, and 244.35 °C in 3 mg/L NO3--containing water, respectively. Correspondingly, the crystallinity of PET microfibers changed from 14.95% to 11.89%, 24.02%, and 15.29% in 3 mg/L Cl-, Br-, and NO3- aquatic solutions, respectively [Supplementary Table 2]. These results indicate that photochemically active ions such as Cl-, Br-, and NO3- play a significant role in accelerating photoaging of PET microfibers in freshwater and seawater.
Figure 5. DSC melting and crystal curves of aged PET at 3 mg/L Cl-, Br-, and NO3--containing aquatic solutions. DSC: Differential scanning calorimetry; PET: polyethylene terephthalate.
Furthermore, GPC was used to analyze the effect of Cl-, Br-, and NO3- on PET microfiber photoaging through changes in the molecular weight of the plastic. As shown in Supplementary Table 3, after 12 days of UV irradiation, the number-average molecular weight (Mn) of PET decreased from 49,718 to 39,120, 43,583, and 27,628 in 3 mg/L Cl-, Br-, and NO3--containing aqueous solutions. Likewise, the weight-average molecular weight (Mw) of PET microfiber decreased from 104,169 to 85,102, 93,224, and 65,850 in the same solutions. These results indicate that UV light irradiation can induce polymer chain scission of PET fibers, thereby decreasing their molecular weight[38,39]. The degree of photoaging of the polyester fibers was then evaluated by the decrease in molecular weight and the increase in the polydispersity index (PDI), which affects properties such as strength, flexibility, and durability[40]. After 12 days of UV exposure, the PDI value increased by 3.67%, 2.05%, and 12.08% in Cl-, Br-, and NO3--containing water, respectively. The increase in PDI also implies that the scission of molecular chains generates a large amount of low-molecular-weight polyester, reducing the average molecular weight of PET fibers and increasing the heterogeneity of the material, potentially affecting its processing and application properties[41]. Notably, the higher increase in the PDI value of PET microfiber during 12 days of photoaging suggests that the presence of NO3- may positively enhance the photoaging process. Therefore, photochemically active ions, including Cl-, Br-, and NO3-, play an important role in controlling the photoaging of PET microfibers in water, directly influencing the diverse photoaging rates in both freshwater and seawater environments.
Mechanistic illustration of the PET microfiber photo-transformation in freshwater and seawater
Photoaging of PET fibers in aqueous environments is driven by direct UV light absorption and free-radical photosensitized oxidation. Therefore, we further explored the effects of photochemically active constituents on PET photoaging. Cl-, Br-, and NO3- have long been recognized as important photochemically active ions that influence the degradation of organic matter, including microplastics, in aquatic systems. Their concentrations in freshwater and seawater samples are provided in Supplementary Table 4[21]. NO3--containing solutions showed a pronounced peak in the 200-400 nm range of the UV-vis spectra [Supplementary Figure 2], whereas Cl- and Br- did not, suggesting that NO3- may more effectively promote PET microfiber photoaging. This is likely related to the resonance structure of NO3-, which enables absorption of specific wavelengths and electronic transitions involving π or delocalized electrons, resulting in enhanced light absorption and the observed spectral peaks[42]. Furthermore, excited-state nitrate ions can transfer energy to dissolved oxygen, generating reactive oxygen species (ROS) with strong oxidizing capacity (e.g. hydroxyl radicals, singlet oxygen, hydrogen peroxide), which further accelerate PET photodegradation[43]. These results indicate that the concentration of inorganic anions is a key factor controlling PET photodegradation[22], and indirect photolysis, particularly anion-induced processes, may play a dominant role. Future studies should examine the specific effects of inorganic anions on PET degradation to better understand its behavior and fate in aquatic environments.
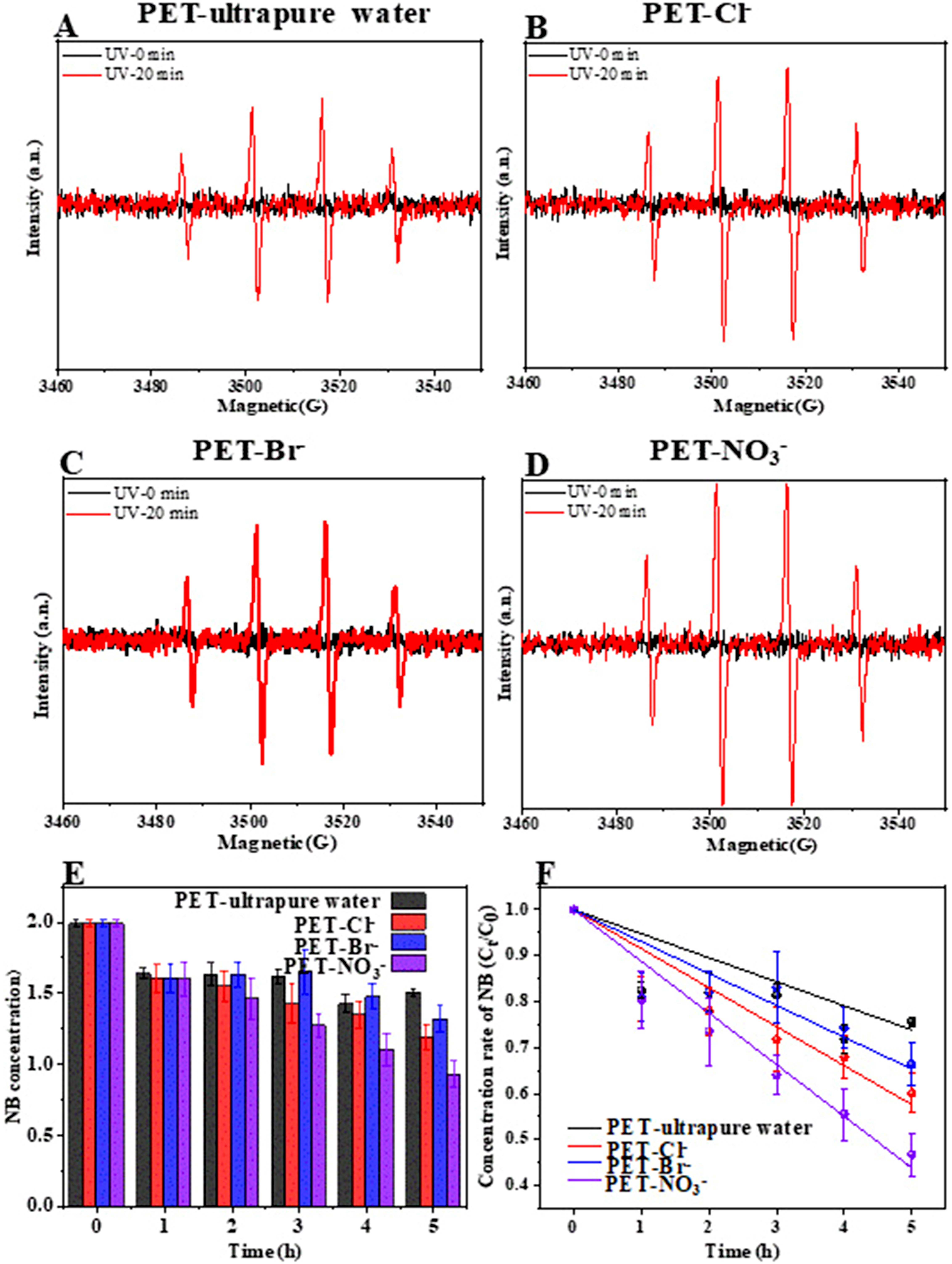
The indirect photoaging of PET is primarily catalyzed by ROS, among which •OH is considered the key reactive species due to its high oxidation-reduction potential (2.8 V)[33,44,45]. As shown in Figure 6A-D, EPR analysis revealed that exposing PET to reaction systems containing ultrapure water, Cl-, Br-, or NO3- under light irradiation generated a characteristic DMPO-OH signal (1:2:2:1), indicating •OH formation. Subsequently, we quantified •OH generated by PET microfiber using nitrobenzene (NB) as a •OH scavenger [Figure 6E and F]. During 5 h of UV irradiation, NB concentration decreased continuously, confirming •OH formation in Cl--, Br--, and NO3--containing solutions. NB degradation followed a pseudo-first-order kinetic model, allowing calculation of the steady-state •OH concentrations during PET photoaging [Supplementary Table 5]. Comparing NB consumption rates, PET fibers in NO3- solutions exhibited the highest rate
Figure 6. (A-D) EPR spectra for the formation of •OH by PET microfiber in Cl-, Br-, and NO3--containing aquatic solutions; (E and F) Quantitative analysis formation of •OH by PET microfiber in Cl-, Br-, and NO3--containing aquatic solutions using NB as capturer. EPR: Electron paramagnetic resonance; PET: polyethylene terephthalate; NB: nitrobenzene.
CONCLUSIONS
In this study, we systematically investigated the photoaging of PET microfibers in freshwater and seawater environments. Results indicated that the photoaging behavior of PET MPs in freshwater and seawater ecosystems was higher than that in ultrapure water, which is preliminarily controlled by co-existing water constituents (i.e. Cl-, Br-, and NO3-). EPR and ROS quantification analysis revealed that the presence of Cl-, Br-, and NO3- can produce 6.04 × 10-15 M, 4.93 × 10-15 M, and 8.00 × 10-15 M •OH, which are higher than that in ultrapure water (3.72 × 10-15 M) and consequently accelerate the photoaging of PET microfibers. These findings help to understand the source and potential environmental behavior of PET microfibers in natural waters, and provide new insights for assessing the ecological risk of PET microplastics in aquatic environments. In the future, it is urgently necessary to investigate how the aging of PET microplastics, which induces changes in surface morphology and physicochemical properties (e.g. roughness, crystallinity), affects the migration of coexisting pollutants in water and the bioavailability of aged PET microfibers to aquatic life. This is vital for systematically assessing the environmental behavior and ecological risks of PET microplastics in aquatic environments.
DECLARATIONS
Authors’ contributions
Conceptualization: Chen, R.; Wu, X.
Writing original draft: Chen, R.; Zhao, X.
Visualization: Chen, R.; Zhao, X.
Writing review and editing: Chen, R.; Wu, X.
Investigation: Zhao, X.; Wu, X.
Validation: Zhao, X.
Methodology: Zhao, X.
Formal analysis: Zhao, X.
Availability of data and materials
The data presented in this study are available from the corresponding author upon reasonable request, due to privacy considerations.
Financial support and sponsorship
This work was financially supported by the National Science Fund for Distinguished Young Scholars of China (42525701), the National Natural Science Foundation of China (22406091), the Natural Science Foundation of Jiangsu Province (BK20240708), the Startup Foundation for Introducing Talent of Nanjing University of Information Science and Technology (2024r064), the Natural Science Research Program of Jiangsu Higher Education Institutions (24KJB610011), and the Guangxi Key Laboratory of Beibu Gulf Marine Resources, Environment, and Sustainable Development (MRESD-2025-A0S).
Conflicts of interest
Wu, X. is a Guest Editor of the Special Issue titled “Interfacial Biodegradation of Plasticizers in (Micro)plastics” in the journal Emerging Contaminants and Environmental Health. Wu, X. was not involved in any steps of the editorial process, notably including reviewers’ selection, manuscript handling, or decision-making. The other authors declare that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2026.
Supplementary Materials
REFERENCES
1. Wang, T.; Zou, X.; Li, B.; et al. Preliminary study of the source apportionment and diversity of microplastics: Taking floating microplastics in the South China Sea as an example. Environ. Pollut. 2019, 245, 965-74.
2. Jiang, Y.; Zhao, Y.; Wang, X.; Yang, F.; Chen, M.; Wang, J. Characterization of microplastics in the surface seawater of the South Yellow Sea as affected by season. Sci. Total. Environ. 2020, 724, 138375.
3. Wang, X.; Zhu, L.; Liu, K.; Li, D. Prevalence of microplastic fibers in the marginal sea water column off southeast China. Sci. Total. Environ. 2022, 804, 150138.
4. Prarat, P.; Hongsawat, P. Microplastic pollution in surface seawater and beach sand from the shore of Rayong province, Thailand: Distribution, characterization, and ecological risk assessment. Mar. Pollut. Bull. 2022, 174, 113200.
5. Kanhai, D. K.; Officer, R.; Lyashevska, O.; Thompson, R. C.; O’Connor, I. Microplastic abundance, distribution and composition along a latitudinal gradient in the Atlantic Ocean. Mar. Pollut. Bull. 2017, 115, 307-14.
6. Pan, Z.; Liu, Q.; Sun, X.; et al. Widespread occurrence of microplastic pollution in open sea surface waters: evidence from the mid-North Pacific Ocean. Gondwana. Res. 2022, 108, 31-40.
7. Hu, L.; Chernick, M.; Hinton, D. E.; Shi, H. Microplastics in small waterbodies and tadpoles from Yangtze River Delta, China. Environ. Sci. Technol. 2018, 52, 8885-93.
8. Kabir, A. H. M. E.; Sekine, M.; Imai, T.; Yamamoto, K.; Kanno, A.; Higuchi, T. Assessing small-scale freshwater microplastics pollution, land-use, source-to-sink conduits, and pollution risks: perspectives from Japanese rivers polluted with microplastics. Sci. Total. Environ. 2021, 768, 144655.
9. Napper, I. E.; Baroth, A.; Barrett, A. C.; et al. The abundance and characteristics of microplastics in surface water in the transboundary Ganges River. Environ. Pollut. 2021, 274, 116348.
10. Chinfak, N.; Sompongchaiyakul, P.; Charoenpong, C.; Shi, H.; Yeemin, T.; Zhang, J. Abundance, composition, and fate of microplastics in water, sediment, and shellfish in the Tapi-Phumduang River system and Bandon Bay, Thailand. Sci. Total. Environ. 2021, 781, 146700.
11. Suaria, G.; Achtypi, A.; Perold, V.; et al. Microfibers in oceanic surface waters: a global characterization. Sci. Adv. 2020, 6, eaay8493.
12. Qiao, R.; Deng, Y.; Zhang, S.; et al. Accumulation of different shapes of microplastics initiates intestinal injury and gut microbiota dysbiosis in the gut of zebrafish. Chemosphere 2019, 236, 124334.
13. Zhang, D.; Cui, Y.; Zhou, H.; et al. Microplastic pollution in water, sediment, and fish from artificial reefs around the Ma’an Archipelago, Shengsi, China. Sci. Total. Environ. 2020, 703, 134768.
14. Jemec, A.; Horvat, P.; Kunej, U.; Bele, M.; Kržan, A. Uptake and effects of microplastic textile fibers on freshwater crustacean Daphnia magna. Environ. Pollut. 2016, 219, 201-9.
15. Cheng, H.; Feng, Y.; Duan, Z.; et al. Toxicities of microplastic fibers and granules on the development of zebrafish embryos and their combined effects with cadmium. Chemosphere 2021, 269, 128677.
16. Jabeen, K.; Li, B.; Chen, Q.; et al. Effects of virgin microplastics on goldfish (Carassius auratus). Chemosphere 2018, 213, 323-32.
17. Sørensen, L.; Groven, A. S.; Hovsbakken, I. A.; et al. UV degradation of natural and synthetic microfibers causes fragmentation and release of polymer degradation products and chemical additives. Sci. Total. Environ. 2021, 755, 143170.
18. Wang, Q.; Zhang, Y.; Wangjin, X.; Wang, Y.; Meng, G.; Chen, Y. The adsorption behavior of metals in aqueous solution by microplastics effected by UV radiation. J. Environ. Sci. 2020, 87, 272-80.
19. Liu, G.; Zhu, Z.; Yang, Y.; Sun, Y.; Yu, F.; Ma, J. Sorption behavior and mechanism of hydrophilic organic chemicals to virgin and aged microplastics in freshwater and seawater. Environ. Pollut. 2019, 246, 26-33.
20. Sharma, M. D.; Krupadam, R. J. Adsorption-desorption dynamics of synthetic and naturally weathered microfibers with toxic heavy metals and their ecological risk in an estuarine ecosystem. Environ. Res. 2022, 207, 112198.
21. He, W.; Liu, S.; Zhang, W.; et al. Recent advances on microplastic aging: identification, mechanism, influence factors, and additives release. Sci. Total. Environ. 2023, 889, 164035.
22. Zhu, K.; Sun, Y.; Jiang, W.; et al. Inorganic anions influenced the photoaging kinetics and mechanism of polystyrene microplastic under the simulated sunlight: role of reactive radical species. Water. Res. 2022, 216, 118294.
23. Deng, Y.; Zhu, K.; Sun, Y.; et al. Aging kinetics and mechanisms of polystyrene microplastic in water under sunlight irradiation: effects of inorganic cations. Gondwana. Research. 2024, 129, 193-200.
24. Wu, X.; Liu, P.; Gong, Z.; et al. Humic acid and fulvic acid hinder long-term weathering of microplastics in lake water. Environ. Sci. Technol. 2021, 55, 15810-20.
25. Huang, W.; Jiang, G.; Xie, L.; Chen, X.; Zhang, R.; Fan, X. Effect of oxygen-containing functional groups on the micromechanical behavior of biodegradable plastics and their formation of microplastics during aging. J. Hazard. Mater. 2024, 463, 132911.
26. Aljoumaa, K.; Abboudi, M. Physical ageing of polyethylene terephthalate under natural sunlight: correlation study between crystallinity and mechanical properties. Appl. Phys. A. 2015, 122, 6.
27. Phan, S.; Padilla-gamiño, J. L.; Luscombe, C. K. The effect of weathering environments on microplastic chemical identification with Raman and IR spectroscopy: Part I. polyethylene and polypropylene. Polym. Test. 2022, 116, 107752.
28. Cai, L.; Wang, J.; Peng, J.; Wu, Z.; Tan, X. Observation of the degradation of three types of plastic pellets exposed to UV irradiation in three different environments. Sci. Total. Environ. 2018, 628-629, 740-7.
29. Hurley, C. R.; Leggett, G. J. Quantitative investigation of the photodegradation of polyethylene terephthalate film by friction force microscopy, contact-angle goniometry, and X-ray photoelectron spectroscopy. ACS. Appl. Mater. Interfaces. 2009, 1, 1688-97.
30. Bai, X.; Li, F.; Ma, L.; Li, C. Weathering of geotextiles under ultraviolet exposure: a neglected source of microfibers from coastal reclamation. Sci. Total. Environ. 2022, 804, 150168.
31. Wang, Z.; Xue, L.; Zhang, Y.; et al. Ubiquitous photoaging of microplastics: potential challenges to air flotation. J. Water. Process. Eng. 2025, 75, 107953.
32. Li, F.; Zhai, X.; Yao, M.; Bai, X. An inevitable but underestimated photoaging behavior of plastic waste in the aquatic environment: critical role of nitrate. Environ. Pollut. 2022, 314, 120307.
33. Duan, J.; Zheng, D.; Wu, Y.; Sleiman, M.; Dong, W. Photoaging of terrestrial plastic pollution: a process affected by precipitation. Environ. Sci. Technol. 2025, 59, 4652-62.
34. Sun, A.; Wang, W. X. Photodegradation controls of potential toxicity of secondary sunscreen-derived microplastics and associated leachates. Environ. Sci. Technol. 2025, 59, 5223-36.
35. Tong, J.; He, S.; Huang, X.; et al. The fate, impacts and potential risks of photoaging process of the microplastics in the aqueous environment. J. Contam. Hydrol. 2025, 275, 104699.
36. Duan, L.; Qin, Y.; Meng, X.; Liu, Y.; Zhang, T.; Chen, W. Sulfide- and UV-induced aging differentially affect contaminant-binding properties of microplastics derived from commercial plastic products. Sci. Total. Environ. 2023, 869, 161800.
37. Zhou, S.; Zhang, W.; Sun, J.; et al. Oxidation mechanisms of the UV/free chlorine process: kinetic modeling and quantitative structure activity relationships. Environ. Sci. Technol. 2019, 53, 4335-45.
38. Shi, Y.; Zheng, L.; Huang, H.; et al. Formation of nano- and microplastics and dissolved chemicals during photodegradation of polyester base fabrics with polyurethane coating. Environ. Sci. Technol. 2023, 57, 1894-906.
39. Cao, Y.; Liu, Y.; Guo, K.; He, W.; Hur, J.; Guo, H. Molecular characteristics and plastic additives in dissolved organic matter derived from polystyrene microplastics: effects of cumulative irradiation and microplastic concentrations. Water. Res. 2025, 282, 123641.
40. Peng, S.; Li, L.; Wei, D.; et al. Releasing characteristics of toxic chemicals from polystyrene microplastics in the aqueous environment during photoaging process. Water. Res. 2024, 258, 121768.
41. Li, L.; Guo, Z.; Guo, X.; et al. Phanerochaete chrysosporium hyphae bio-crack, endocytose and metabolize plastic films. J. Hazard. Mater. 2025, 487, 137154.
42. Kang, Y. M.; Kim, M. K.; Zoh, K. D. Effect of nitrate, carbonate/bicarbonate, humic acid, and H2O2 on the kinetics and degradation mechanism of Bisphenol-A during UV photolysis. Chemosphere 2018, 204, 148-55.
43. Zhang, X.; Li, J.; Fan, W. Y.; Sheng, G. P. Photomineralization of effluent organic phosphorus to orthophosphate under simulated light illumination. Environ. Sci. Technol. 2019, 53, 4997-5004.
44. Ma, W.; He, J.; Han, L.; et al. Hydrophilic fraction of dissolved organic matter largely facilitated microplastics photoaging: insights from redox properties and reactive oxygen species. Environ. Sci. Technol. 2024, 58, 11625-36.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Topic
Copyright
Data & Comments
Data















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.