Zeolite-based catalysts for the synthesis of γ-valerolactone from various biomass feedstocks
Abstract
The growing demand for sustainable alternatives to fossil fuels has increased interest in biomass-derived chemicals, which play a vital role in the global transition toward renewable energy. Among these chemicals, γ-valerolactone (GVL) stands out as a promising intermediate for producing high-value chemicals, including bio-diesel. Zeolites, with their exceptional stability and catalytic activity, have demonstrated remarkable performance in GVL synthesis, making them highly suitable as heterogeneous catalysts for targeted biomass conversion. This review explores the mechanisms and methods employed in the preparation of these catalysts for converting various biomass feedstocks into GVL. Additionally, we discuss recent progress in zeolite-catalyzed GVL production from common biomass sources. Finally, we address the challenges and future prospects for developing more effective zeolite-based catalysts in GVL synthesis.
Keywords
INTRODUCTION
Fossil fuels are essential to the global economy, supporting both economic development and national security. However, rising concerns about climate change, potential oil crises due to geopolitical conflicts in oil-producing regions, and the desire of many nations to reduce dependence on oil products have prompted researchers to seek alternative energy sources to replace fossil fuels[1,2].
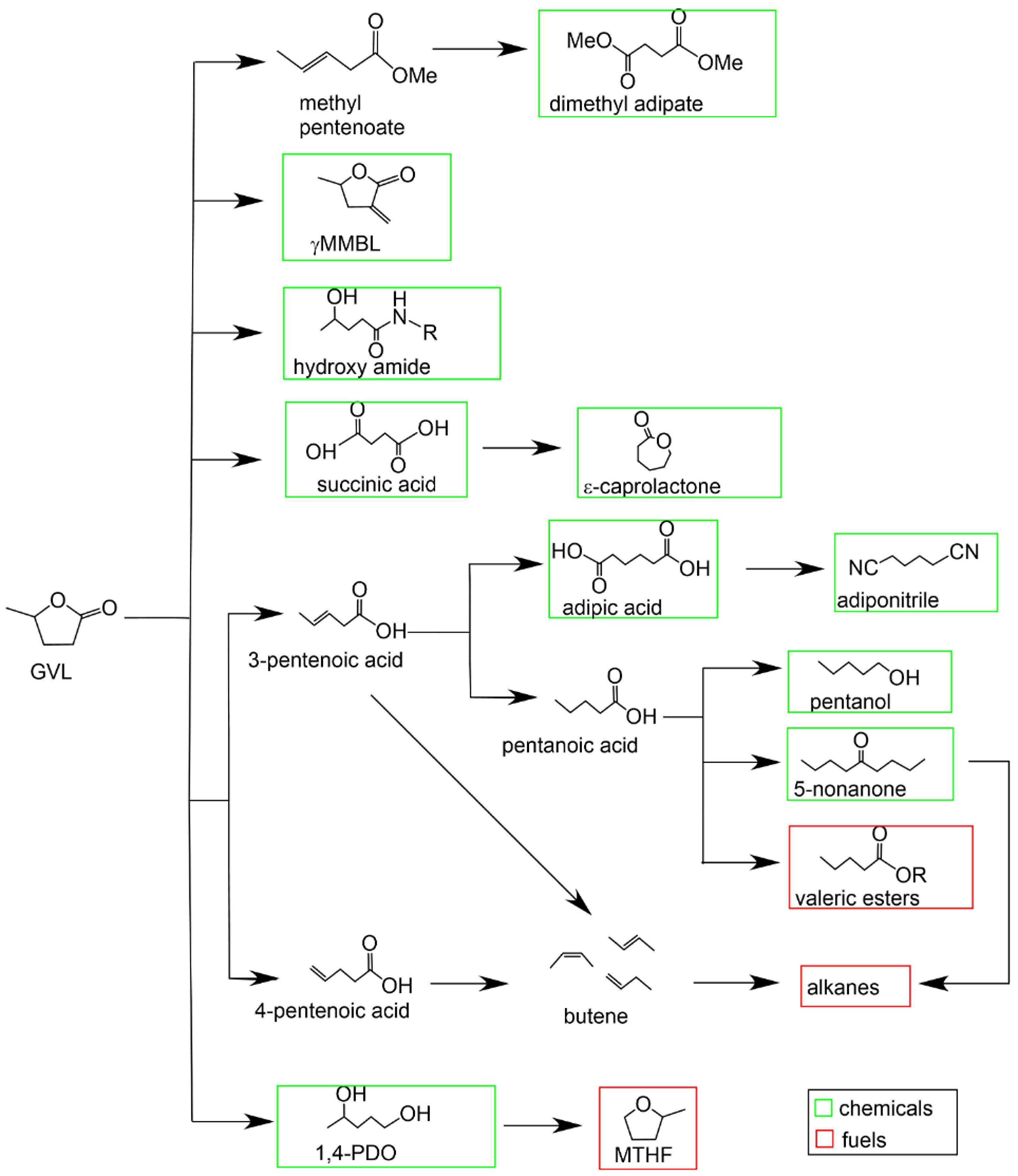
Lignocellulosic biomass, a natural renewable resource, can be transformed into various valuable compounds such as furfural (FUR), furfural alcohol (FAL), levulinic acid (LA), ethyl levulinate (EL) and γ-valerolactone (GVL), which can be then further processed into biofuels and other important industrial chemicals[3]. Among these compounds, GVL serves as a key intermediate for valeric acid biofuels, which can be blended with diesel or gasoline in high proportions[4,5]. It can be converted into butene mixtures, which are polymerized into C8-C16 oligomeric olefins and subsequently hydrogenated to form alkanes. Additionally, GVL can be selectively transformed into pentanoic acid, which undergoes ketonization to yield 5-nonanone - a precursor for gasoline-range C9-C18 alkanes[6]. Moreover, it can be further processed into valeric acid esters, regarded as next-generation biofuels, with valeric acid serving as an intermediate[3] [Figure 1].
Figure 1. Conventional pathway for the conversion of GVL to fuels and chemicals. Reproduced with permission[3]. Copyright John Wiley and Sons. GVL: γ-Valerolactone.
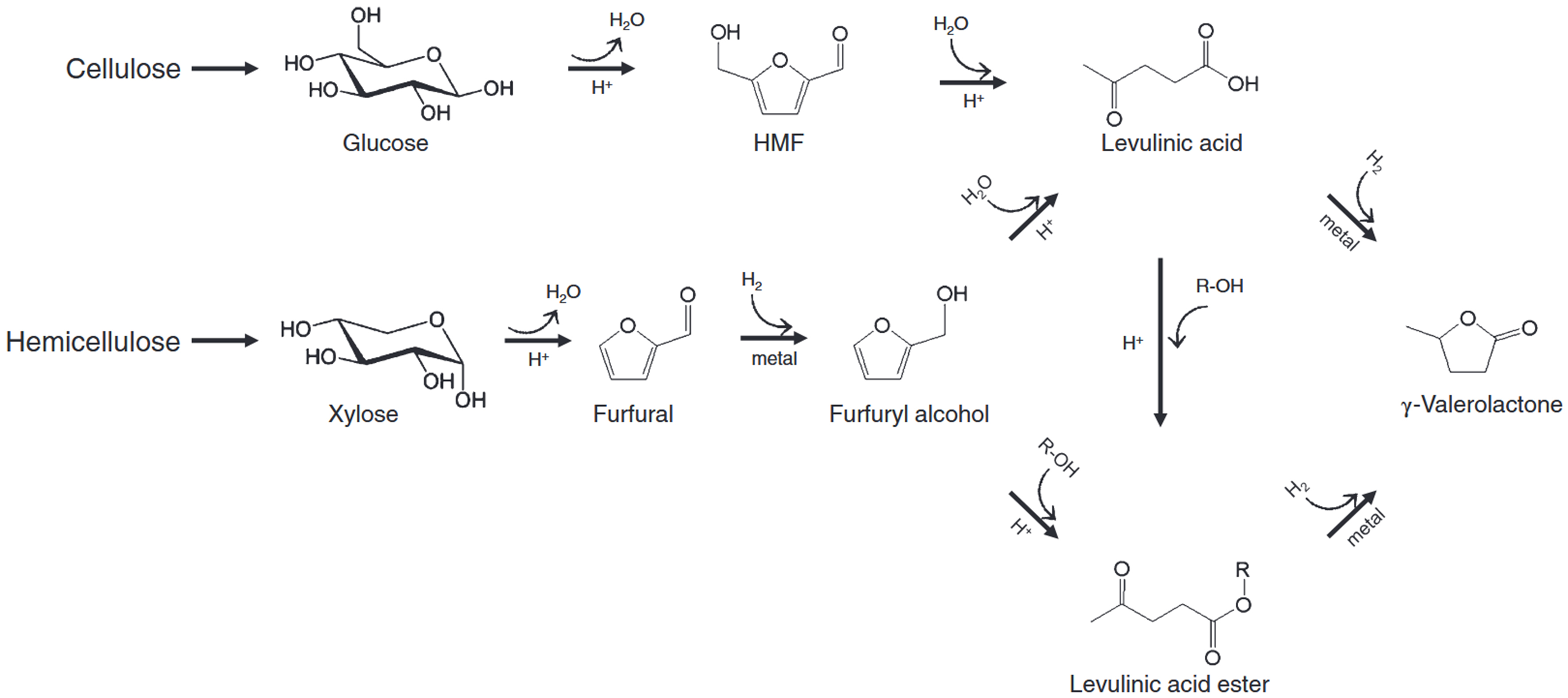
Several pathways exist for GVL synthesis[7] [Figure 2]. For example, Yu et al. reported that GVL can be produced from various biomass-derived feedstocks, including LA, EL, and FUR, through processes such as hydrolysis, dehydration, and reduction[6]. Stable intermediates allow for either stepwise reactions or one-pot synthesis with multiple catalysts. Unstable intermediates, however, require sequential transformations with specific catalysts for each step[8-10].
Figure 2. Conventional pathways to synthesize GVL from biomass feedstocks. Reproduced with permission[7]. Copyright John Wiley and Sons. GVL: γ-Valerolactone.
The conversion of lignocellulosic biomass compounds, i.e., lignin, cellulose, and hemicellulose, and their derived platform compounds, into high-value chemicals primarily involves dehydration and hydrodeoxygenation (HDO)[11]. Dehydration removes oxygen as water without altering carbon oxidation states, typically using acid-base chemistry[12]. HDO, in contrast, reduces the oxygen content with hydrogen donors, often favoring hydrogen due to its availability and ease of activation on metal surfaces. However, safety concerns about high-pressure hydrogen have led researchers to explore liquid-phase hydrogen donors[11].
Many oxygen-rich biomass molecules have high boiling points and are susceptible to decomposition when evaporated, so biomass upgrading is frequently conducted in the liquid phase using solvents. The low solubility of hydrogen in these solvents necessitates high hydrogen pressures for efficient conversion. Certain organic molecules, however, can serve as renewable hydrogen donors in catalytic transfer hydrogenation (CTH)[13], reducing the need for high-pressure hydrogen. Using liquid organic hydrogen donors mitigates the safety risks associated with hydrogen gas, improves solubility in liquid-phase reactions, and simplifies the experimental setups. Additionally, the lower hydrogenation capacity of these donors compared to hydrogen enhances selectivity for partially biomass molecules.
Detailed theoretical studies and kinetic experiments have advanced our understanding of GVL synthesis. Ju et al. investigated the mechanistic pathways of GVL synthesis using isopropanol-aluminum catalysts and M062X-D3 computations. They demonstrated that the positively charged Al center forms a strong hydrogen bond with the ester carbonyl group, stabilizing a tetradentate cyclic intermediate during ester exchange[14]. Additionally, they identified the ring-closure step as the rate-determining step for GVL formation. Isopropanol was found to have a higher proton transport rate compared to other solvents, reducing the reaction energy barrier from 46.9 kcal/mol (uncatalyzed) to 37.4 kcal/mol. Similarly, kinetic experiments using Ru/C catalysts confirmed that LA facilitates the cyclization step, further promoting GVL formation[15-17].
Lewis acid sites are critical for hydrogenation during GVL synthesis, while Brønsted acid sites play a key role in macromolecular dehydration and hydrolysis[18]. Therefore, both types of acidity must be considered when designing effective catalysts. Over the past few years, various homogeneous catalysts have been developed for GVL synthesis[19,20]. These catalysts provide easy access to active catalytic sites. For example, Shvo catalysts are commonly used in reactions involving the hydrogenation of LA to an intermediate 4-hydroxyvaleric acid, followed by cyclization to produce GVL[20]. Formic acid is frequently employed as a hydrogen donor, producing 4-hydroxyvaleric acid and CO2.
Although homogeneous catalysts exhibit excellent catalytic performance and have been extensively studied, they are more suitable for biomass conversion due to their non-corrosive nature, ease of separation, and recyclability - key attributes for sustainable and green chemistry. Various acidic solid catalysts, including metal-organic frameworks, zeolites, sulfonated polymers, and resins, have been developed for biomass conversion.
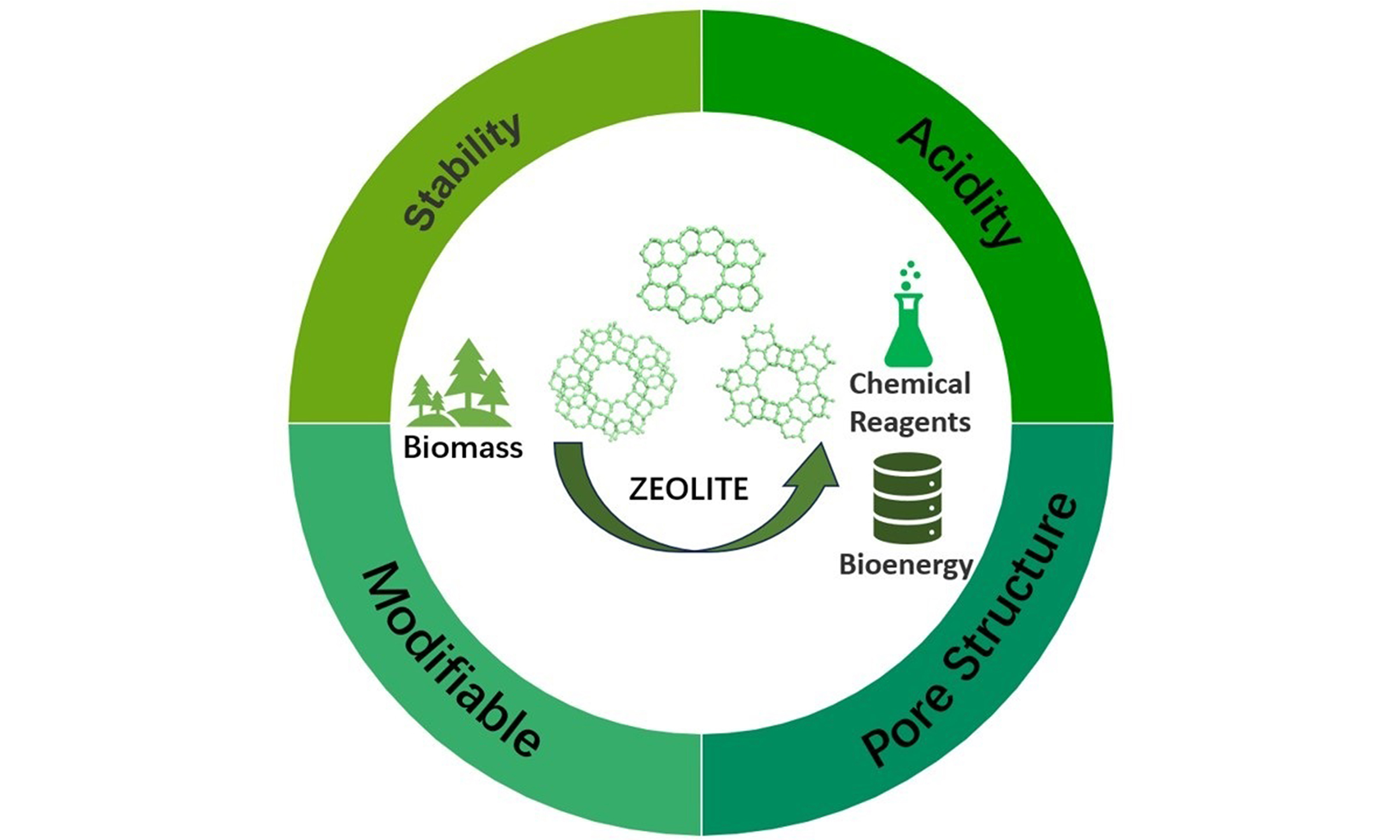
Zeolites, in particular, are ideal catalysts due to their high stability, specific surface area, multi-cycle usability, and tunable active sites for enhanced Lewis acidity[21] [Figure 3]. They are crystalline aluminosilicates, made of interconnected TO4 (T = Al, Si, P, etc.) tetrahedra, forming a three-dimensional framework with molecular-sized pores and cages. With diverse T atom types and complex TO4 arrangements, over 260 distinct zeolite structures have been recognized by the International Zeolite Association. Zeolites are widely used in catalysis, gas adsorption and separation, and ion exchange[22-25]. Among these structures, FAU and Beta, with twelve-membered ring pores, are extensively used for GVL synthesis due to their ability to facilitate reactions involving large organic molecules. Additionally, zeolites with ten-membered ring pores, such as MFI and MWW, have been reported as effective for GVL synthesis.
Figure 3. Summary of the characteristics of zeolite-based catalysts in the transfer of biomass feedstocks.
Several methods have been developed for zeolite modification to enhance their catalytic performance: (1) Impregnation: This widely used method involves mixing a zeolite carrier with a metal precursor solution, followed by drying and calcination to convert the precursor into metal or metal oxide[26,27]. Impregnation enables the confinement of precious metals (e.g., Pd, Pt, Au) or metal anions (e.g., WO42-) in the zeolite framework to achieve high catalytic efficiency; (2) Ion-exchange: This technique leverages the negatively charged zeolite framework, where extra-framework cations (e.g., Na+, K+, NH4+, H+, etc.) can be exchanged with catalytic active cations in solution[28,29]; (3) Atomic layer deposition (ALD): ALD allows for the precise deposition of metal precursors at the atomic level[30-33]. This involves loading a degassed zeolite into a vacuum chamber, pulsing a vaporized metal precursor to react with surface groups, and purging unreacted species with inert gas. Repeated cycles increase metal loading; (4) In situ encapsulation or in situ confinement synthesis: This method involves introducing metal precursors during zeolite synthesis or transformation to achieve ultrasmall, well-dispersed metal clusters[34]; (5) Physical mixing: A straightforward approach involving the mechanical mixing of zeolites with Lewis acid catalysts to produce efficient composite catalysts without complex chemical reactions[35].
Significant advances in GVL synthesis have been summarized in recent reviews. However, most focus on general catalysts rather than zeolites specifically[3,6,36], or they discuss GVL synthesis within broader contexts[9,37]. This review highlights recent advancements in zeolite-catalyzed valorization of biomass feedstocks for GVL production. We explore different reaction routes utilizing LA, EL, FUR, FAL, and saccharides as feedstocks, detailing the mechanisms and catalytic roles of zeolites. Finally, we discuss the benefits and limitations of zeolite-based GVL synthesis, along with future prospects and challenges for zeolite catalysts in this field.
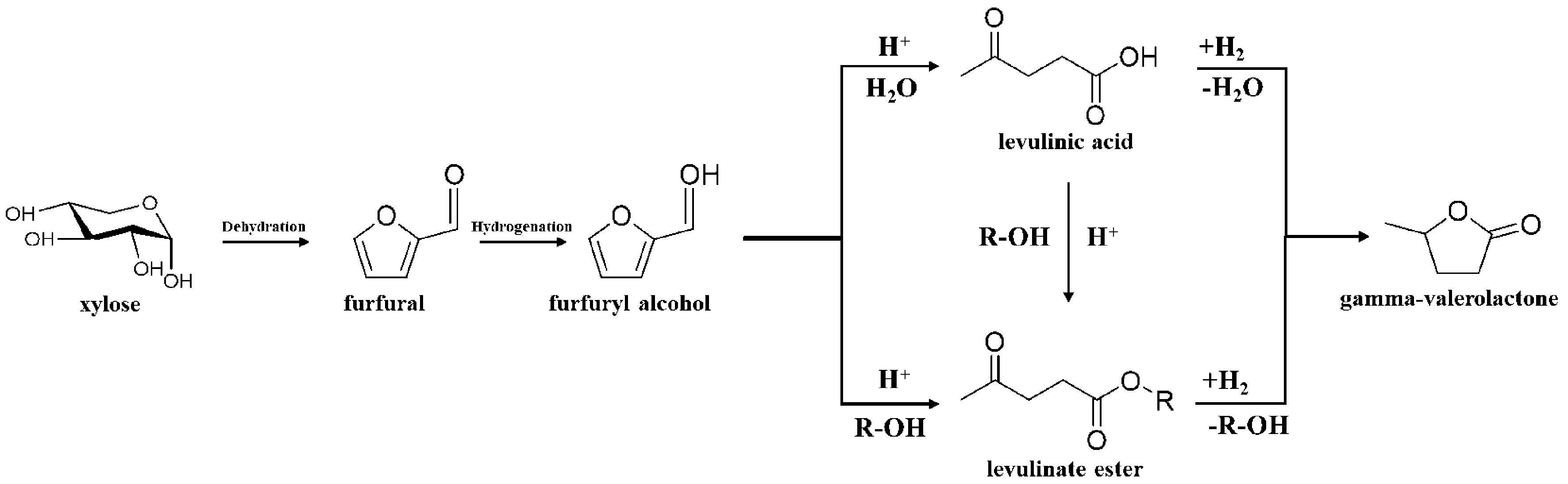
SYNTHESIS OF GVL FROM LA AND ITS ESTERS
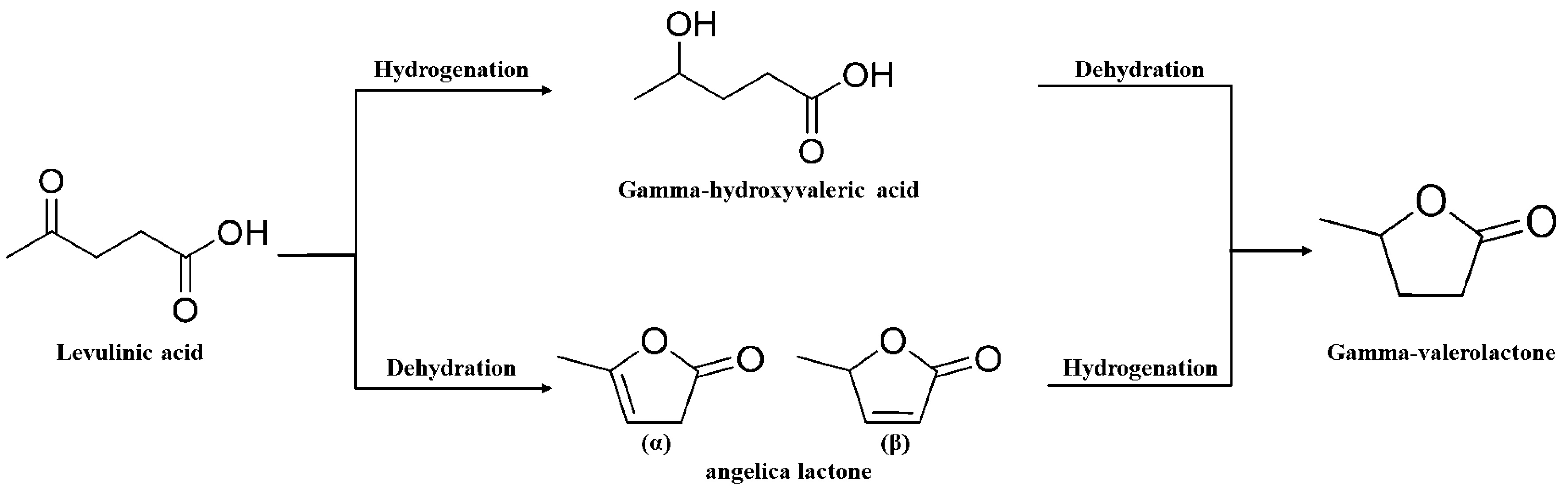
LA and its esters are important platform chemicals derived from lignocellulosic biomass[38]. They can be converted into a wide range of valuable products, including fuel additives, synthetic polymer monomers, and other chemicals. The primary pathway for GVL synthesis involves catalytic conversion of LA and its esters[3]. This process typically includes: (1) Hydrogenation of carbonyl groups: The carbon-oxygen double bonds in the carbonyl groups of LA and its esters are hydrogenated, forming γ-hydroxyvaleric acid or γ-hydroxyvalerate as intermediates; (2) Intramolecular dehydration and esterification: These intermediates undergo esterification via intramolecular dehydration to form GVL. An alternative pathway for GVL synthesis involves the enolization of LA’s carbonyl group at high temperatures (> 180 °C), leading to the formation of α-angelica lactone via intramolecular esterification. The hydrogenation of α-angelica lactone then yields GVL. Notably, levulinate esters are more reactive than LA under similar conditions, producing higher GVL yields and facilitating a simpler separation process. This advantage arises from the reduced causticity of levulinate esters compared to LA.
Synthesis of GVL from LA
In 2004, the U.S. Department of Energy identified LA as one of the top 12 high-value biobased platform compounds[4]. LA can be synthesized from various biomass resources, making it a highly promising candidate for sustainable chemical production[6]. Its two oxygen-containing functional groups - carbonyl and carboxyl - enable its conversion into several high-value chemicals through different reaction pathways, including esterification to produce alkyl acetyl propionate, hydrogenation to form GVL, and reductive amination to synthesize pyrrolidinone[6].
For GVL synthesis, LA undergoes hydrogenation to form γ-hydroxyvaleric acid or its ester, followed by intramolecular dehydration to yield GVL. At temperatures above 180 °C, LA can enolize and form α-angelicolide lactone via intramolecular esterification, which is subsequently hydrogenated to GVL[6] [Figure 4].
Figure 4. Reaction mechanism of GVL synthesis from LA. Reproduced with permission[6], Copyright Elsevier. GVL: γ-Valerolactone; LA: levulinic acid.
When using zeolite catalyst for LA-to-GVL conversion, modifications are often necessary to enhance dehydration, hydrolysis, hydrogenation, and cyclization, as zeolites typically lack the Lewis acid sites needed for the reaction[37]. Metal-loaded zeolites, for instance, enhance LA hydrogenation by providing active hydrogenation sites and modifying the zeolite acidity[39] [Table 1]. The use of zeolite catalysts for LA-to-GVL conversion has garnered significant attention in recent studies, as this reaction is a crucial step in GVL synthesis. Moreover, insights from these studies can guide the development of strategies for producing GVL from other biomass-derived feedstocks.
A summary of recent research progress in the synthesis of GVL using LA as the starting material
| Catalyst | Hydrogen donor | Temp. (°C) | Time (h) | Yield (%) | Selec. (%) | Ref. |
| Beta | ||||||
| Zr-Beta-100 | 2-pentanol | 118 | 10 | 96 | 96 | [40] |
| Zr-Al-Beta | Isopropanol | 170 | 6 | ~90 | ~90 | [41] |
| Zr-Al-Beta | Isopropanol | 170 | 6 | 90 | 95 | [42] |
| Hf-WdeSiAlBeta-m | 2-butanol | 180 | 24 | 91 | 91 | [43] |
| Beta@LDH-OV0.15 | Isopropanol | 220 | 14 | 89.2 | 89.2 | [44] |
| MFI | ||||||
| Ru/ZSM-5M | H2 | 70 | 6 | 95 | 95.3 | [45] |
| ZSM-5-3Ni | H2 | 320 | 4 | 98.6 | 100 | [46] |
| PtNi3@S-1(TCPP) | H2 | 180 | 6 | 89.8 | 93.8 | [47] |
| Ru/ZSM(40)-5 | H2 | 100 | 1 | 98 | 100 | [48] |
| ZKD-5(11) | H2 | 130 | 5 | 99 | 100 | [49] |
| Pt0.7-Ce0.5/TS-1 | H2 | 180 | 6 | 99.4 | 99.8 | [50] |
| Ni-ZSM-5(IMP) | H2 | 275 | 10 | 63.4 | 91 | [51] |
| 3Ni-HZSM | H2 | 220 | 10 | 93.1 | > 98.5 | [52] |
| Zr/NaSP | Isopropanol | 180 | 1.5 | 83.1 | 83.1 | [53] |
| FAU | ||||||
| 3PtY | H2 | 220 | 24 | 92 | 92 | [54] |
| ZrY | Formic acid | 220 | 24 | 66 | 71 | [55] |
| Zr1Y1 | H2 | 220 | 10 | 95 | 99 | [56] |
| 2.6Pt/Y-C18TAOH-2 | H2 | 120 | 2 | 94 | 94 | [57] |
| TY700 | Isopropanol | 175 | 12 | 94 | 94% ± 2% | [58] |
| Pt-Ru(1:0.25)/Y | H2 | 160 | 6 | 98 | 98 | [59] |
| AEI | ||||||
| Pt/AIPO-18(5CMC) | H2 | 160 | 2 | 93.2 | 97.9 | [60] |
| 2%Ru/H-SSZ-39 | H2 | 150 | 4 | 95.4 | 98.3 | [61] |
| MWW | ||||||
| Ru/MCM-49(DP) | H2 | 160 | 0.5 | 94.3 | 99 | [62] |
| Ru-Mn(0.7)/MCM-49 | H2 | 160 | 3 | 98 | 100 | [63] |
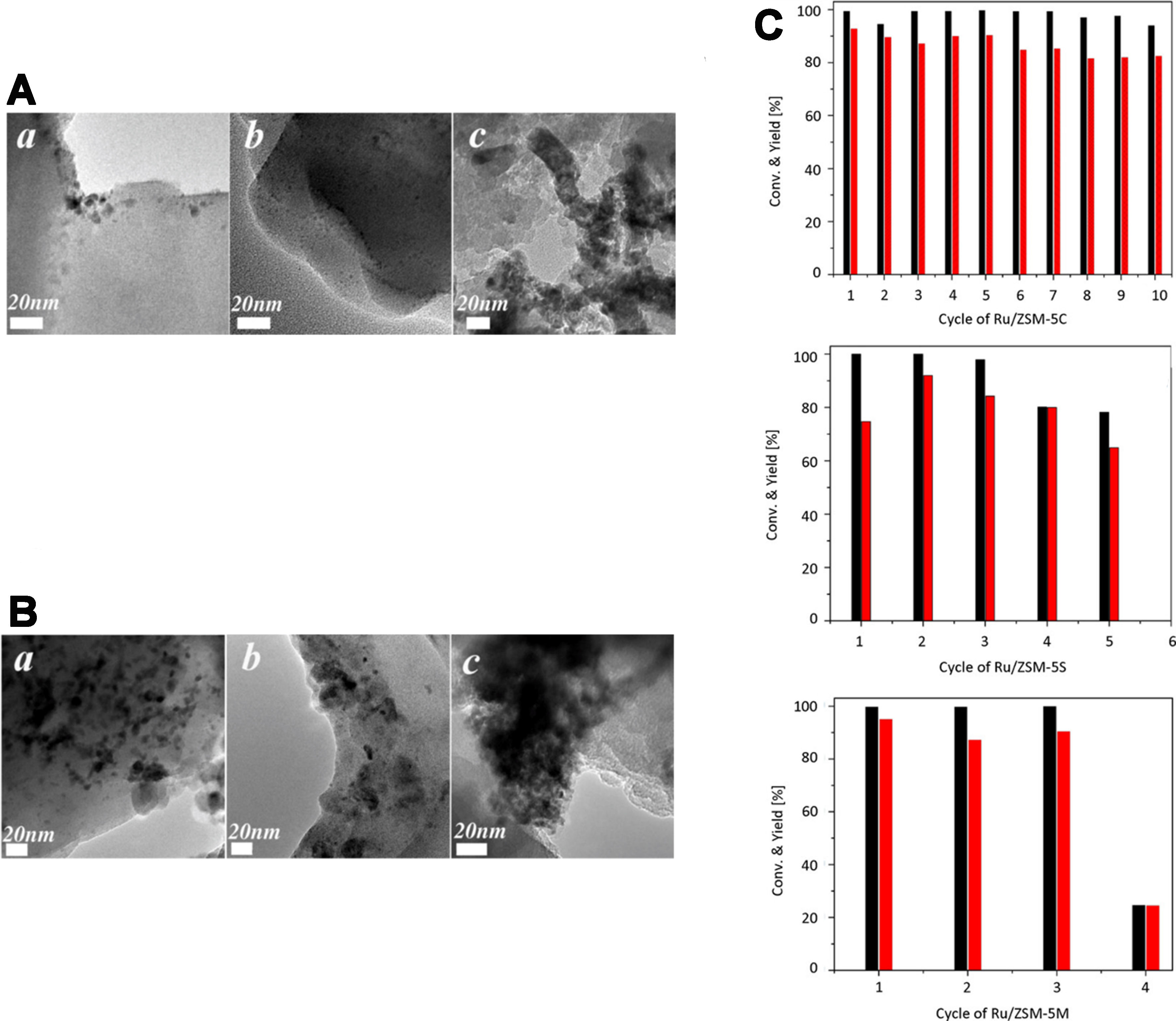
Noble metals such as Ru, Rh, Pd, Pt, Au, and Re demonstrate high catalytic efficiency in LA hydrogenation, outperforming other metals such as Cu, Ni, and Co[64]. However, these catalysts tend to deactivate rapidly. For example, Li et al. reported that Ru/MCM-49 catalysts showed particle agglomeration and carbon buildup after ten cycles, leading to reduced activity[62].
Zhang et al. developed Ru/ZSM-5 hydrogenation catalysts via various preparation methods for LA hydrogenation in water[45]. Their Ru/ZSM-5 catalyst, prepared by wet impregnation, achieved 95% LA conversion and 85% GVL yield. The presence of tetra-coordinated Al species on the ZSM-5 surface prevented Ru leaching and enhanced stability, as confirmed by 27Al magic angle spinning-nuclear magnetic resonance (MAS NMR) and X-ray photoelectron spectroscopy (XPS) analyses. The low pore volume and interconnected channels of the ZSM-5 support also minimized carbon residue formation, maintaining stable performance over ten consecutive cycles[45] [Figure 5]. In another study, Al-Khawlani et al. prepared Pt-Ru/Y zeolite catalysts using ethylene glycol reduction for GVL synthesis in a tetrahydrofuran (THF) solvent[59]. These catalysts achieved a 100% LA conversion and a 90.12% GVL yield at a low Pt-to-Ru ratio (1:0.25). NaOH treatment improved catalyst performance by adjusting the Si/Al ratio, enhancing Pt-Ru dispersion on the zeolite surface and within its pores. Consequently, the treated catalyst exhibited superior hydrogenation activity, achieving 100% LA conversion and an improved GVL yield of 97.99%. Moreover, it retained the structural integrity of the Y zeolite even after multiple reaction cycles, demonstrating high stability.
Figure 5. (A) TEM images of three fresh catalysts: (a) Ru/ZSM-5C; (b) Ru/ZSM-5S; (c) Ru/ZSM-5M. (B) TEM images of the cycled catalysts: (a) Ru/ZSM-5C; (b) Ru/ZSM-5S; (c) Ru/ZSM-5M. (C) Recycling results for Ru/ZSM-5 catalysts in the hydrogenation of LA. Black: LA conversion and red: GVL yield. Reproduced with permission[45]. Copyright John Wiley and Sons. TEM: Transmission electron microscopy; LA: levulinic acid; GVL: γ-valerolactone.
For noble metal-based catalysts, rapid deactivation and coking can often be mitigated through specific preparation methods, such as mixing the metal with zeolite or modifying zeolite acidity to ensure uniform metal dispersion within the zeolite pores. However, the high cost of precious metals such as Ru, Pt, and Pd has motivated research into alternatives[7].
Recent studies have focused on non-precious metals such as Zr, Cu, and Ni, which offer similar activity and selectivity to noble metals[64]. Zeolites doped with tetra-coordinated atoms such as Zr, Sn, and Hf introduce Lewis acid sites that promote lactonization. For instance, Sn- and Zr-Beta zeolites with open sites exhibit increased reactivity due to higher Lewis acid density[65]. These Lewis acid sites significantly influence the selectivity and conversion. By optimizing and designing these sites, the performance of catalysts can be further improved, enabling efficient reactions under mild conditions, thus advancing the green synthesis of GVL.
Popova et al. investigated the effect of varying the Ni/Al ratios on the catalytic properties of Ni/HZSM-5[46]. They characterized the catalyst using techniques such as X-ray powder diffraction, scanning electron microscopy with energy-dispersive X-ray spectroscopy (SEM-EDX), transmission electron microscopy with energy-dispersive X-ray spectroscopy (TEM-EDX), pyridine temperature-programmed desorption (TPD), and diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS), hydrogen temperature-programmed reduction (H2-TPR), N2 physical adsorption, and isoelectric point measurements. As the nickel content increased, the number of Brønsted acid sites decreased, while the number of Lewis acid sites rose. The catalyst exhibited optimal performance when the balance between Lewis and Brønsted acid sites was achieved, and the reducibility of NiO active sites reached approximately 80%. Under these conditions, the catalyst achieved 99% LA conversion and 100% GVL selectivity, highlighting the importance of a balanced ratio of Lewis and Brønsted acid sites for maximizing GVL yield.
In addition to Ni-based catalysts, Cu-based catalysts have also shown promise in LA hydrogenation to GVL. Liang et al. developed Cu-based ZKD-5 (MFI) catalysts by adjusting the Cu/Si ratio[49]. This created a range of Cu active sites, including secondary framework Cu in the 10MR ring and quasi sub-nano cluster Cu in the zeolite cage. Kinetic experiments and modeling revealed that secondary framework Cu sites adsorb carbonyl groups, while quasi sub-nano cluster and secondary framework Cu sites activate hydrogen. The strong metal-support interactions provided by the zeolite framework enhanced the stability of the Cu catalyst. The ZKD-5 catalyst achieved 100% selectivity and conversion at a low reaction temperature of
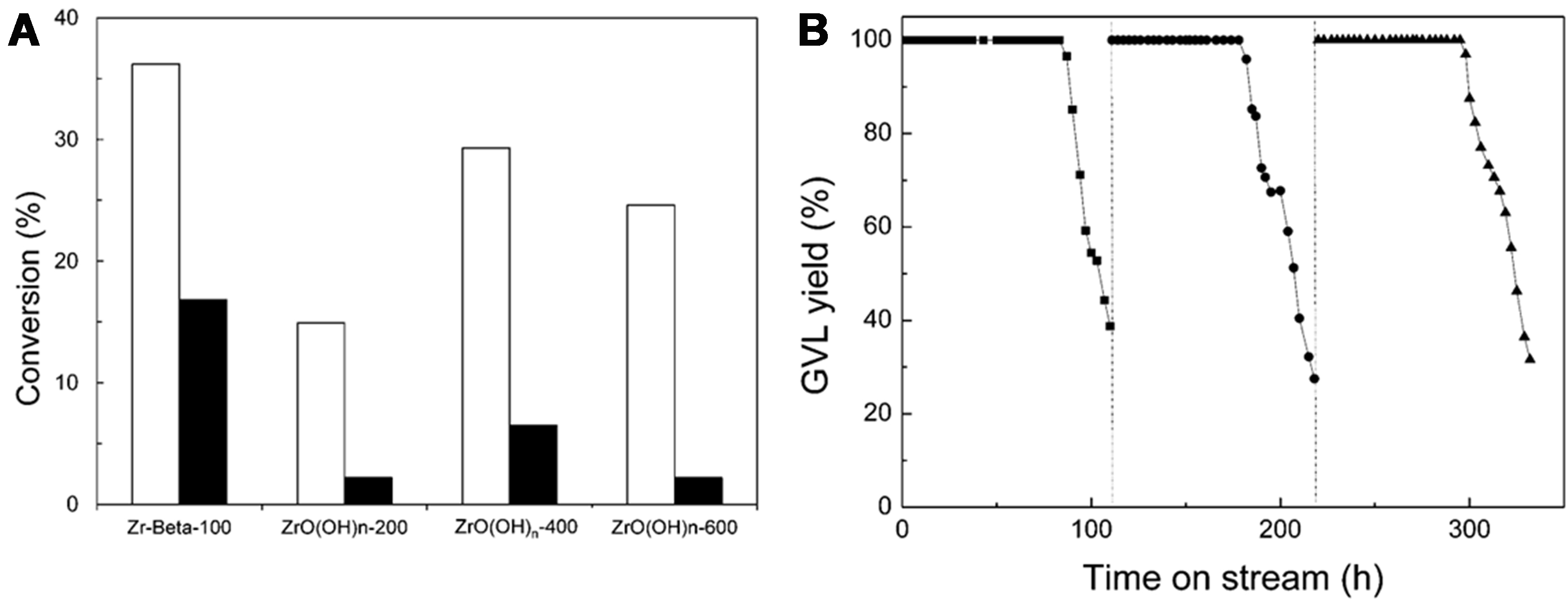
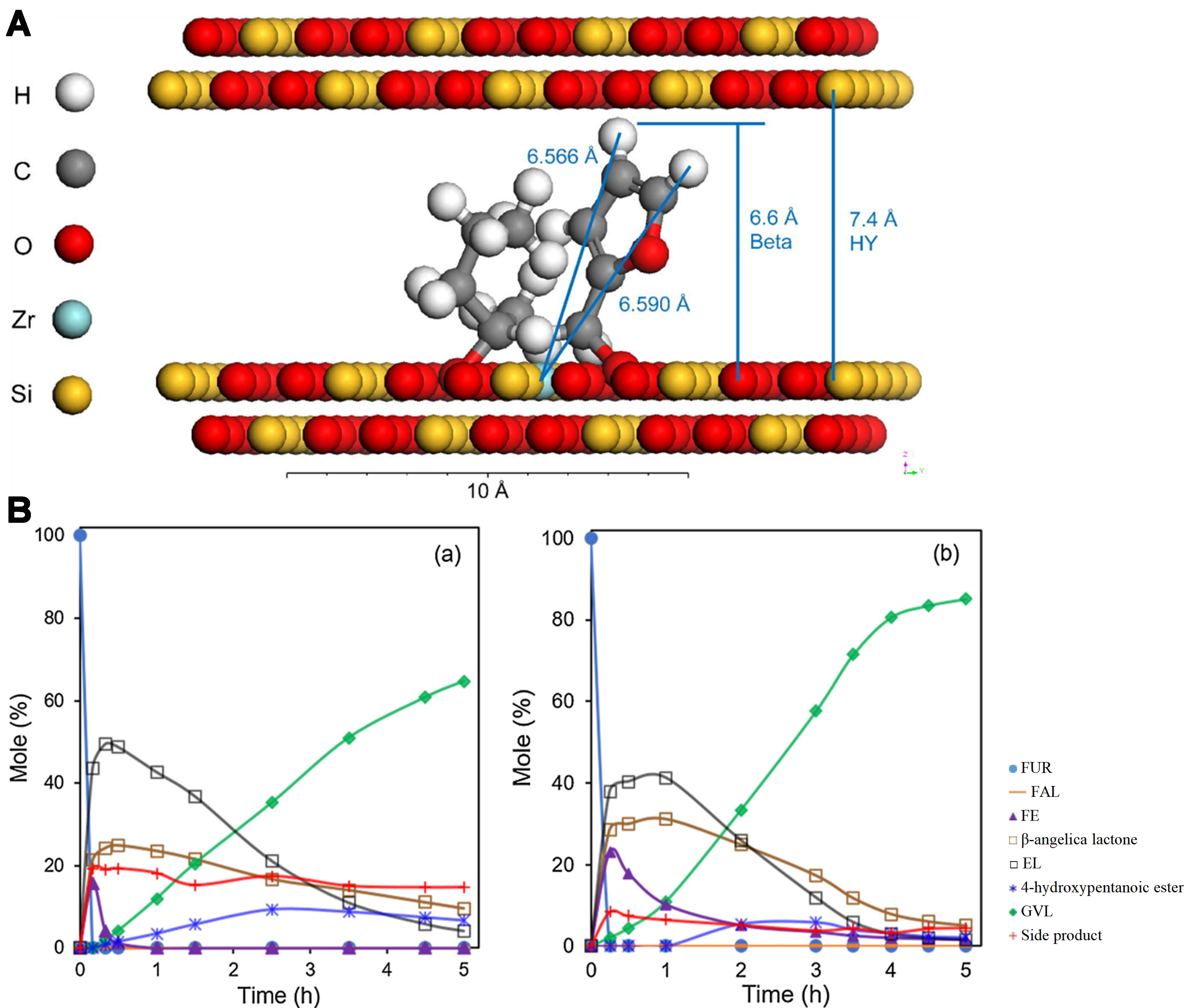
Zr-based catalysts are also widely used in biomass conversion and have proven highly effective in GVL synthesis when combined with zeolites. Wang et al. synthesized highly stable and reactive Zr-Beta and Zr-Al-Beta zeolites, achieving over 96% GVL selectivity in batch reactors and more than 99% yield in continuous flow reactors[40]. The high activity is attributed to moderately strong Lewis acid sites, which are resistant to deactivation by acidic reactants. This stability enables their long-term use with minimal performance degradation[40] [Figure 6].
Figure 6. (A) Conversion of methyl levulinate (□) and LA (■) to GVL after 2 h using Zr-Beta-100 and ZrO(OH)n-T as catalysts. Reaction conditions included 1 mmol of substrate, 5 mL of 2-pentanol, 200 mg of catalyst, and a temperature of 118 °C; (B) GVL yield as a function of time for the MPV reaction of LA over Zr-Beta-100 at 250 °C in a continuous flow reactor, with a WHSV of 0.64 h-1. The catalyst was regenerated at 110 and 218 h. Reproduced with permission[40]. Copyright Royal Society of Chemistry. LA: Levulinic acid; GVL: γ-valerolactone; MPV: Meerwein-Ponndorf-Verley; WHSV: weight hourly space velocity.
Synthesis of GVL from EL
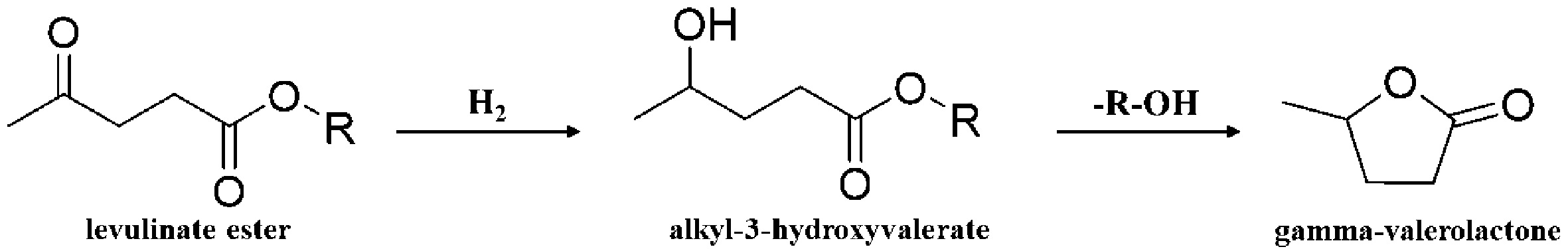
EL, derived from LA, is a promising compound with potential applications as a diesel or biodiesel additive. EL improves the cloud point, pour point, and cold filter plugging point of biodiesel fuels with high saturated fatty acid content[66,67]. Additionally, the oxygen content in EL promotes more complete combustion, potentially reducing particulate emissions from incomplete fuel combustion. The pathway for GVL synthesis from EL is similar to that of LA hydrogenation, with intermediates undergoing esterification and cyclization to form GVL [Figure 7]. Moreover, they are ideal substrates for the synthesis of GVL under reducing conditions, and their separation process is relatively straightforward, further enhancing their utility as raw materials for GVL production[6]. It should be noted that there are many types of levulinate esters, but here we just list EL as a reference due to little difference in the reactions of these compounds.
Figure 7. Reaction mechanism of GVL synthesis from levulinate ester. Reproduced with permission[6], Copyright Elsevier. GVL: γ-Valerolactone.
Similar to LA, the hydrogenation of EL can be catalyzed using zeolite-based catalysts modified with precious or tetra-coordinated metal [Table 2]. Chen et al. prepared a series of Pt-containing zeolite catalysts via wet impregnation and evaluated their performance for EL hydrogenation in ethanol under hydrogen[72]. Among the catalysts tested, 1% Pt/ZSM-35 exhibited the highest activity, achieving 100% EL conversion and 99.0% GVL selectivity under optimized conditions (200 °C, 6 MPa H2). Stability tests indicated that the catalyst maintained high activity for GVL production even after three consecutive cycles. Tang et al. synthesized hierarchical FAU-type hafnosilicate zeolite with a hierarchical structure (Hf-USY) zeolites using a post-synthesis strategy[71]. Due to the high electron affinity of Hf, the six-membered transition state during the hydrogen transfer step was more stable than that of catalysts containing Zr or Sn. Under reaction conditions of 413 K, the Hf-USY catalyst achieved 95.1% EL conversion and 92.9% GVL selectivity. In addition to the widely studied Zr-based catalysts, various approaches using other metals as active sites are also being investigated. Regardless of the metal used, optimizing the acid-base sites remains crucial for improving GVL yield. Lu et al. investigated the hydrothermal synthesis of Sn-Beta catalysts for EL hydrogenation under mild conditions[68]. While high temperatures are typically needed for high catalytic activity, the microenvironment of Sn species within the zeolite framework significantly influences the catalytic properties. For example, Sn-Beta catalysts synthesized over 7 days or longer showed higher crystallinity and increased framework Sn content. These factors result in an increased number of Lewis acid sites, both of which enhanced EL hydrogenation.
A summary of recent research progress in the synthesis of GVL using EL as the starting material
SYNTHESIS OF GVL FROM XYLOSE, FUR, AND FAL
Xylose, FUR, and FAL are pivotal intermediates in the conversion of biomass hemicellulose to gentian violet. Their conversion is of significant interest in the field of sustainable chemistry due to their potential to facilitate efficient GVL production via the hemicellulose pathway. The production of GVL from Fur and FAL is more complex, with more challenging reaction steps and more demanding reaction conditions than those involved in the reaction pathway for LA and its esters[73,74]. Hemicellulose can be hydrolyzed to xylose using dilute acid (typically H2SO4), which is then further hydrolyzed to FUR[75]. This can then undergo further hydrogenation to FAL, which can be converted to LA and its esters through hydrolysis. In the presence of Brønsted acid, an alcoholysis reaction is carried out, and GVL can finally be synthesized from LA and its esters[76-78]. The one-pot method for the production of GVL from FUR/FAL as a starting material involves a number of series of steps. LA and its esters are often considered to be the key intermediates in the cascade process. The conversion of LA and its esters to GVL has been previously discussed in this section. This multi-step conversion involves a tandem reaction, and the choice of catalysts, reaction pathways, and process optimization play a crucial role in determining the overall efficiency and product distribution.
Synthesis of GVL from xylose
Xylose, a five-carbon sugar derived from hemicellulose, is highly soluble and has a low heat of combustion, making it an attractive bio-based feedstock for GVL synthesis. After dehydration to FUR, it undergoes further reactions to produce GVL[10] [Figure 8].
Figure 8. Reaction mechanism of one-pot GVL synthesis from xylose/FUR/FAL. Reproduced with permission[6]. Copyright Elsevier. GVL: γ-Valerolactone; FUR: furfural; FAL: furfural alcohol.
However, synthesizing GVL from xylose using zeolite catalysts remains challenging, with limited research and suboptimal catalytic performance [Table 3]. To enhance the conversion efficiency, researchers often modify the ratio of Brønsted to Lewis acid sites (B/L) in zeolite catalysts[79]. For instance, Li et al. investigated the direct conversion of xylose to GVL using Zr-Al-SCM-1 catalysts, adjusting the B/L[81]. They observed a volcano-shaped relationship between the B/L ratio and GVL yield, with the highest GVL yield of 36.4% achieved at a B/L ratio of 0.46 under optimized conditions. Similarly, Hernández et al. used a tunable Al/Zr ratio in Zr-Al-Beta zeolite for the one-pot conversion of xylose to GVL[80]. They achieved a yield of up to 35% at 190 °C after 48 h, using a catalyst with an Al/Zr ratio of 0.22.
A summary of recent research progress in the synthesis of GVL using xylose as the starting material
Despite these advances, the conversion efficiency of xylose to GVL remains relatively low. This is primarily due to the large size of xylose molecules and the complexity of the cascade reactions involved in the process. Further optimization of active sites or the use of mixed catalyst systems is necessary to enhance the conversion efficiency at each reaction step.
Synthesis of GVL from FUR
FUR is an industrial chemical derived from agricultural by-products such as corn cobs, wheat bran, and sawdust. Due to its abundance in biomass feedstocks, FUR is considered a promising direct feedstock for GVL production. However, converting FUR to GVL involves complex cascade reactions, including CTH, etherification, ring-opening, partial hydrogenation, and cyclization[83]. This reaction pathway is more intricate than the conversion of LA or its esters, requiring more steps and stricter reaction conditions.
In the primary pathway for FUR conversion to GVL[6] [Figure 7], FUR is first reduced to FAL over metal catalysts. Under acidic conditions, FAL partially converts to furfuryl ether (FE). Both FAL and FE undergo Brønsted acid-catalyzed alcoholysis to form acetyl propionate. Most acetyl propionate is then converted to 4-hydroxyvaleric acid via Lewis acid catalysis, while a smaller fraction forms furanone. Finally, 4-hydroxyvaleric acid and furanone undergo lactonization to form GVL. Due to the complexity of this multi-step process, highly active catalysts with optimized structures and acid-base properties are crucial for efficient GVL synthesis[10]. Many zeolites meeting these criteria have been studied [Table 4]. Recent studies on using FUR as a feedstock for GVL synthesis have shown mixed results, largely due to the multiple reactions involved. Achieving optimal B/L ratios and suitable pore structures in the catalysts is critical for reducing carbon deposits and improving overall efficiency.
A summary of recent research progress in the synthesis of GVL using FUR as the starting material
| Catalyst | Hydrogen donor | Temp. (°C) | Time (h) | Yield (%) | Selec. (%) | Ref. |
| Beta | ||||||
| (Sn)SSIE-Beta1 | 2-butanol | 120 | 48 | 73 | 82 | [74] |
| Sn-Al-Beta 7 | 2-butanol | 180 | 24 | 60.5 | 60.5 | [84] |
| Meso-Zr-Al-Beta | Isopropanol | 120 | 24 | 95 | 95 | [85] |
| Zr-Al-Beta | Isopropanol | 170 | 24 | 71 | 70 | [86] |
| HPW/Zr-Beta | Isopropanol | 160 | 24 | 68 | 68 | [87] |
| ZrO2-TPA-Beta zeolite | Isopropanol | 170 | 10 | 90 | 90 | [88] |
| 0.8Al/Zr-Beta(29) | Isopropanol | 160 | 24 | 93.3 | 93.3 | [89] |
| Zr/Beta-CEL | Isopropanol | 180 | 8 | 81.1 | 81.1 | [90] |
| ZrP@HB-4(4) | Isopropanol | 170 | 8 | 64.2 | 64.5 | [91] |
| MFI | ||||||
| Au/ZrO2 + ZSM-5 | Isopropanol | 120 | 30 | 80.4 | 80.4 | [92] |
| ZrO2-[Al]MFI-NS 30 | Isopropanol | 170 | 36 | 82.8 | 82.8 | [93] |
| Ni-Co-Fe/ZSM-5 | Ethanol | 150 | 14 | 85.7 | 85.7 | [94] |
| 1% Pt/ZSM-5 | Isopropanol | 120 | 10 | 87.8 | 91.7 | [95] |
| ZrO2/NS + Zr(OH)4-0.03g | Isopropanol | 180 | 2 | 97.1 | > 97.1 | [96] |
| FAU | ||||||
| Zr-HY-15-20 and Al-HY-15 | 2-pentanol | 120 | 1 | 94 | 99 | [97] |
| 5% Hf-Al-USY | Isopropanol | 140 | 12 | 64.9 | 64.9 | [98] |
| SnZr/Y (1:1) | Isopropanol | 180 | 2 | 90 | 90 | [99] |
| TY700 | Isopropanol | 175 | 12 | 52 | 52 | [100] |
| Combo zeolite | ||||||
| Zr-Beta + Al-MFI-ns | 2-butanol | 120 | 48 | 78 | - | [101] |
One effective strategy to enhance catalytic performance is to combine catalytically active substances with zeolites. Mixed-component catalysts are widely used in heterogeneous catalytic processes due to their superior thermal and chemical stability compared to single-component catalysts. García et al. reported improved results using Sn- and Zr-based catalysts supported on treated Y zeolites, compared to monometallic catalysts[99]. This bimetallic catalyst, with a Sn:Zr ratio of 1:1, achieved optimal performance, yielding 80% GVL after just 1 h at 180 °C. This high performance is attributed to the broad dispersion of metal species on the carrier surface, which forms a mixed dispersion of Zr-O-Sn species. This dispersion enhances the availability of both Lewis and Brønsted acid sites, promoting efficient catalysis. Additionally, the catalyst demonstrated excellent stability, maintaining consistent GVL yields over at least four cycles. This stability is likely due to the strong and stable interaction between the surface metal species and the carrier.
Modulating acid sites and mesopores can significantly enhance catalytic performance. Acid sites improve catalytic efficiency, while mesopores help reduce side reactions. Gao et al. developed a magnetically recoverable multifunctional catalyst by loading ZrO2 onto mesoporous MCM-41-coated Fe3O4 nanoparticles[102]. The incorporation of Fe3O4 not only imparted magnetic properties for easy catalyst recovery but also modified the acidity to promote GVL production. Using isopropanol as both the hydrogen donor and solvent, this catalyst achieved an 85% GVL yield.
Recently, researchers have focused on adjusting zeolite acid sites to reduce metal usage and lower costs. Zhang et al. prepared a combined zeolite catalyst by physically mixing Zr-HY and Al-HY[97]. At an optimal weight ratio of 2:1 (Zr-HY to Al-HY), the catalyst exhibited excellent activity for the one-pot cascade reaction, achieving an 85% GVL yield after 5 h at 120 °C. This catalyst could be easily recycled and reused for at least three cycles without significant loss in activity or GVL yield[97] [Figure 9]. Jayakumari et al. explored the regulation of zeolite acid sites through controlled dealumination by heat treatment[100]. As the treatment temperature increased, the number of strong acid sites decreased due to the removal of Al from the framework, while the number of weak acid sites increased. 27Al MAS NMR studies revealed that heating Y zeolite from 500 to 700 °C generated more five-coordinated Al and extra-framework octahedral Al sites. Despite the reduction in total acid sites, this catalyst achieved 100% FUR conversion and a 52% GVL yield under optimal conditions. The catalysts could be recycled three times without loss of catalytic activity, and regeneration via heat treatment in air at 500 °C restored its performance to that of a fresh catalyst. Post-treatment de-alumination of zeolites, using conventional high-temperature steam or acid etching, can significantly increase GVL yields.
Figure 9. (A) Model showing the spatial constraints for coordination of FUR and 2-pentanol at zirconium Lewis acid sites within the pore channel; (B) Reaction profiles for one-pot conversion of FUR to GVL with simultaneous addition of (a) Zr-HY-15-20 and Al-HY-15 and (b) Zr-HY-15-20 and Al-HY-6. Reaction conditions: 0.20 g Zr-HY-15-20, 0.10 g Al-HY-6, 1 mmol FUR, 5 mL 2-pentanol, at 120 °C under N2. Reproduced with permission[97]. Copyright Elsevier. FUR: Furfural; GVL: γ-valerolactone.
Synthesis of GVL from FAL
FAL, a hydroxymethyl-substituted furan derivative, is synthesized by hydrogenating FUR or via a one-pot method from xylose. As previously discussed, FAL is an important intermediate in the conversion of FUR to GVL. The catalysts effective for FUR conversion can also facilitate the transformation of FAL into GVL. With ongoing improvements in the production of FAL from xylose[103], it is becoming increasingly relevant to investigate its direct conversion to GVL using zeolites.
A large part of the research on the zeolite-catalyzed conversion of FAL to GVL overlaps with the research on the conversion of FUR. Here we only list some of the studies using FAL as the substrate. Wang et al. proposed an efficient process for producing both LA and GVL from FAL, using GVL as a self-circulating solvent[104]. They tested various zeolites, the mixtures of metal oxide and zeolite, and several homogeneous catalysts. Among the zeolites tested - H-Y, H-β, USY, MCM-22, and ZSM-5 - fixed-bed catalytic systems consistently outperformed autoclave setups. In particular, H-ZSM-5 achieved a 99.9% conversion rate for LA with a 90.5% yield, which was approximately 20% higher than in the autoclave. When H-ZSM-5 was combined with Ru/ZrO2 for the direct one-step conversion of FAL to GVL, side reactions occurred, resulting in a GVL yield of only 48.5% in the autoclave. By-products such as 2-methyltetrahydrofuran and tetrahydrofurfuryl alcohol were also detected. However, in the fixed-bed system, the GVL yield increased to 89.4%, with minimal by-products.
Although zeolites with tunable properties are widely used for FAL conversion with excellent catalytic performance, separating the catalyst from the reaction medium often involves labor-intensive operations. Lima et al. performed a valorization reaction of FAL into high-value chemicals using a core-shell magnetic ZSM-5 zeolite[105]. By using 2-propanol as both solvent and hydrogen donor at 130 °C, they achieved 100% FAL conversion and 97% selectivity for GVL, with only a slight decrease in activity after four reuse cycles. Moreover, the magnetic ZSM-5 zeolite enabled easy separation from the reaction medium, simplifying the overall process.
SYNTHESIS OF GVL FROM SACCHARIDES
Saccharides, naturally occurring polyhydroxy aldehydes or ketones, include monosaccharides, disaccharides, and polysaccharides. They are abundant, renewable, and have drawn increasing attention for applications beyond their traditional role as energy sources[6,7].
Cellulose, the most abundant biopolymer in nature, can be catalytically transformed into valuable derivatives[6] [Figure 10]. However, due to its large molecular size, cellulose struggles to react within the small pores of conventional zeolites, resulting in limited reports on its catalytic conversion. Karanwal et al. explored a one-pot conversion of cellulose in supercritical methanol using a Ru-Cu/Y zeolite catalyst[106]. The reaction yielded 90% cellulose conversion and 49.8% GVL, with a catalyst having a Si/Al ratio of 40. This reaction was conducted under supercritical methanol conditions at 250 °C, 3 MPa hydrogen pressure, and a 5 h reaction time. The development of extra-large pore zeolites, such as ZEO-1[107], could enable the catalytic conversion of macromolecules such as cellulose within these zeolites.
Figure 10. Reaction mechanism of one-pot GVL synthesis from saccharides. Reproduced with permission[6]. Copyright Elsevier. GVL: γ-Valerolactone.
Glucose, derived from cellulose hydrolysis, is another abundant and accessible feedstock. Recent advancements have enabled its conversion to GVL through a one-pot multi-step process. However, similar to cellulose, glucose’s six-carbon structure limits its access to zeolite pores, resulting in fewer studies. Paniagua et al. investigated the one-pot conversion of glucose to GVL by adjusting the Al/Zr ratio in Zr-Al-Beta catalysts[108]. They found that a higher Al/Zr ratio favored FUR production, whereas lowering the ratio enhanced GVL formation. The optimal catalyst achieved a GVL yield of about 50% at an Al/Zr ratio of 0.2, representing the best performance in their catalyst series.
Cellulose-derived saccharides are more readily available than many other feedstocks and offer excellent potential for lowering the cost of GVL synthesis. However, their large molecular size prevents most carbohydrate analogs from fitting into zeolite pores. Even relatively small monosaccharides exist in multiple isomeric forms and remain in dynamic equilibrium in solution. This complexity makes it challenging for a single catalytic site to process them effectively.
CONCLUSION AND OUTLOOK
Zeolite catalysts have shown broad applicability in biomass conversion processes for the production of GVL. Topologies such as FAU, MFI, Beta, and MWW have been widely studied. Their tunable Brønsted and Lewis acid sites - achieved by adjusting the Si/Al ratio or introducing additional metal species by physical mixing, impregnation, ion exchange, and in-situ encapsulation or deposition - are critical for biomass valorization. Furthermore, their porous structures, with large specific surface areas, provide an abundance of catalytic sites. In addition, the large pores of twelve-membered ring zeolites, such as FAU and Beta, facilitate the diffusion of the reactants, enhancing their suitability for this reaction. Unlike other heterogeneous catalysts, zeolites resist humin formation and can be regenerated through high-temperature treatment, making them highly recyclable. However, for zeolites to become more suitable for biorefineries as they transition from conventional refineries, several optimizations are required:
(1) Reduced metal use: Hydrogenation using hydrogen remains the standard industrial method for GVL production. This process achieves higher product concentrations but consumes significant amounts of precious metal catalysts and carries safety risks. In contrast, using alcohol as a hydrogen donor ensures safer production and allows for the use of non-precious metals, such as Zr- or Hf-based zeolite materials, which have shown catalytic performance comparable to that of precious metal catalysts in alcohol systems[83]. Further exploration of metal-free methods, such as optimizing acid site ratios or doping metal-based catalysts with conventional zeolite materials, holds promise for reducing costs and resource dependence[58,96,100].
(2) Adapting pore structures: As biomass feedstocks become more diverse, the pore structures of existing zeolites may not accommodate certain large biomolecules, complicating catalytic reactions[82]. Developing zeolites with larger pore sizes or modifying extra-large pore zeolites[109], such as ZEO-1[107], for biomass conversion could address this limitation. Additionally, incorporating mesopores can reduce side reactions. Mesopores can be introduced by adding surfactants such as cetyltrimethylammonium bromide (CTAB) during synthesis or using the crystal seed method.
(3) Rational regulation of active sites: Side reactions during GVL synthesis can reduce overall efficiency. Careful regulation of active sites - whether Brønsted/Lewis acid sites or combinations of metal and Brønsted acid sites - can improve the carbon balance and maximize feedstock utilization.
In summary, zeolite materials have significant potential for GVL production from biomass, but further improvements in their catalytic performance are essential. The zeolite-catalyzed approaches reviewed here offer valuable insights and are expected to guide future developments in this field.
DECLARATIONS
Authors’ contributions
Prepared and revised the manuscript: Wang, C.
Revised the manuscript: Hou, P.
Designed and revised the manuscript: Yan, W.
All authors contributed to the discussion and preparation of the manuscript.
Availability of data and materials
Not applicable.
Financial support and sponsorship
This work was supported by the National Key Research and Development Program of China (2021YFA1500401), the National Natural Science Foundation of China (22288101), and the “111 Center” (B17020).
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Johnson, N. C.; Xie, S. P.; Kosaka, Y.; Li, X. Increasing occurrence of cold and warm extremes during the recent global warming slowdown. Nat. Commun. 2018, 9, 1724.
2. Asif, M.; Muneer, T. Energy supply, its demand and security issues for developed and emerging economies. Renew. Sustain. Energy. Rev. 2007, 11, 1388-413.
3. Xu, R.; Liu, K.; Du, H.; et al. Falling leaves return to their roots: a review on the preparation of γ-valerolactone from lignocellulose and its application in the conversion of lignocellulose. ChemSusChem 2020, 13, 6461-76.
4. U.S. Department of Energy. Top value added chemicals from biomass. Volume I - results of screening for potential candidates from sugars and synthesis gas. https://docs.nrel.gov/docs/fy04osti/35523.pdf. (accessed 9 May 2025)
5. Lange, J. P.; Price, R.; Ayoub, P. M.; et al. Valeric biofuels: a platform of cellulosic transportation fuels. Angew. Chem. Int. Ed. Engl. 2010, 49, 4479-83.
6. Yu, Z.; Lu, X.; Liu, C.; Han, Y.; Ji, N. Synthesis of γ-valerolactone from different biomass-derived feedstocks: recent advances on reaction mechanisms and catalytic systems. Renew. Sustain. Energy. Rev. 2019, 112, 140-57.
7. Osatiashtiani, A.; Lee, A. F.; Wilson, K. Recent advances in the production of γ-valerolactone from biomass-derived feedstocks via heterogeneous catalytic transfer hydrogenation. J. Chem. Technol. Biotech. 2017, 92, 1125-35.
8. Alonso, D. M.; Wettstein, S. G.; Dumesic, J. A. Gamma-valerolactone, a sustainable platform molecule derived from lignocellulosic biomass. Green. Chem. 2013, 15, 584.
9. Li, H.; Yang, S.; Riisager, A.; et al. Zeolite and zeotype-catalysed transformations of biofuranic compounds. Green. Chem. 2016, 18, 5701-35.
10. Wang, J.; Xiang, Z.; Huang, Z.; Xu, Q.; Yin, D. Recent advances on bifunctional catalysts for one-pot conversion of furfural to γ-valerolactone. Front. Chem. 2022, 10, 959572.
11. Gilkey, M. J.; Xu, B. Heterogeneous catalytic transfer hydrogenation as an effective pathway in biomass upgrading. ACS. Catal. 2016, 6, 1420-36.
12. Chheda, J. N.; Dumesic, J. A. An overview of dehydration, aldol-condensation and hydrogenation processes for production of liquid alkanes from biomass-derived carbohydrates. Catal. Today. 2007, 123, 59-70.
13. Johnson, T. C.; Morris, D. J.; Wills, M. Hydrogen generation from formic acid and alcohols using homogeneous catalysts. Chem. Soc. Rev. 2010, 39, 81-8.
14. Ju, Z.; Feng, S.; Ren, L.; et al. Probing the mechanism of the conversion of methyl levulinate into γ-valerolactone catalyzed by Al(OiPr)3 in an alcohol solvent: a DFT study. RSC. Adv. 2022, 12, 2788-97.
15. Piskun, A.; van de Bovenkamp, H.; Rasrendra, C.; Winkelman, J.; Heeres, H. Kinetic modeling of levulinic acid hydrogenation to γ-valerolactone in water using a carbon supported Ru catalyst. Appl. Catal. A. Gen. 2016, 525, 158-67.
16. Delgado, J.; Vasquez Salcedo, W. N.; Bronzetti, G.; et al. Kinetic model assessment for the synthesis of γ-valerolactone from n-butyl levulinate and levulinic acid hydrogenation over the synergy effect of dual catalysts Ru/C and Amberlite IR-120. Chem. Eng. J. 2022, 430, 133053.
17. Salcedo, W. N. V.; Mignot, M.; Renou, B.; Leveneur, S. Assessment of kinetic models for the production of γ-valerolactone developed in isothermal, adiabatic and isoperibolic conditions. Fuel 2023, 350, 128792.
18. Yan, K.; Yang, Y.; Chai, J.; Lu, Y. Catalytic reactions of gamma-valerolactone: a platform to fuels and value-added chemicals. Appl. Catal. B. Environ. 2015, 179, 292-304.
19. Mehdi, H.; Fábos, V.; Tuba, R.; Bodor, A.; Mika, L. T.; Horváth, I. T. Integration of homogeneous and heterogeneous catalytic processes for a multi-step conversion of biomass: from sucrose to levulinic acid, γ-valerolactone, 1,4-pentanediol, 2-methyl-tetrahydrofuran, and alkanes. Top. Catal. 2008, 48, 49-54.
20. Fábos, V.; Mika, L. T.; Horváth, I. T. Selective conversion of levulinic and formic acids to γ-valerolactone with the shvo catalyst. Organometallics 2014, 33, 181-7.
21. Xu, R.; Pang, W.; Yu, J.; Huo, Q.; Chen, J. Chemistry of zeolites and related porous materials:synthesis and structure. John Wiley & Sons (Asia) Pte Ltd; 2007.
22. Wang, Y.; Han, J.; Jin, K.; et al. Fluoride-free synthesis of high-silica RHO zeolite for the highly selective synthesis of methylamine. Inorg. Chem. Front. 2024, 11, 5473-83.
23. Su, H.; Zhou, Q.; Jin, K.; et al. Ultra-low silica zeolite TON: facile synthesis and efficient catalysis in n-dodecane hydroisomerization. Fuel 2024, 376, 132651.
24. Wang, B.; Li, J.; Zhou, X.; et al. Facile activation of lithium slag for the hydrothermal synthesis of zeolite A with commercial quality and high removal efficiency for the isotope of radioactive 90Sr. Inorg. Chem. Front. 2022, 9, 468-77.
25. Wang, X.; Yan, N.; Xie, M.; et al. The inorganic cation-tailored “trapdoor” effect of silicoaluminophosphate zeolite for highly selective CO2 separation. Chem. Sci. 2021, 12, 8803-10.
26. He, P.; Chen, Y.; Jarvis, J.; et al. Highly selective aromatization of octane over Pt-Zn/UZSM-5: the effect of Pt-Zn interaction and Pt position. ACS. Appl. Mater. Interfaces. 2020, 12, 28273-87.
27. Li, L.; Liu, S.; Jiang, R.; et al. Subnanometric Pt on Cu nanoparticles confined in Y-zeolite: highly-efficient catalysts for selective catalytic reduction of NOx by CO. ChemCatChem 2021, 13, 1568-77.
28. Ryu, T.; Ahn, N. H.; Seo, S.; et al. Fully copper-exchanged high-silica LTA zeolites as unrivaled hydrothermally stable NH3-SCR catalysts. Angew. Chem. Int. Ed. Engl. 2017, 56, 3256-60.
29. Lin, W. C.; Wu, S.; Li, G.; et al. Cooperative catalytically active sites for methanol activation by single metal ion-doped H-ZSM-5. Chem. Sci. 2020, 12, 210-9.
30. Sree, S. P.; Dendooven, J.; Korányi, T. I.; et al. Aluminium atomic layer deposition applied to mesoporous zeolites for acid catalytic activity enhancement. Catal. Sci. Technol. 2011, 1, 218.
31. Jiang, F.; Huang, J.; Niu, L.; Xiao, G. Atomic layer deposition of ZnO thin films on ZSM-5 zeolite and its catalytic performance in chichibabin reaction. Catal. Lett. 2015, 145, 947-54.
32. Xu, D.; Wang, S.; Wu, B.; et al. Highly dispersed single-atom Pt and Pt clusters in the Fe-modified KL zeolite with enhanced selectivity for n-heptane aromatization. ACS. Appl. Mater. Interfaces. 2019, 11, 29858-67.
33. Zhang, J.; Lu, Z.; Wu, W.; et al. Mesopore differences between pillared lamellar MFI and MWW zeolites probed by atomic layer deposition of titania and consequences on photocatalysis. Micropor. Mesopor. Mat. 2019, 276, 260-9.
34. Kulkarni, A.; Lobo-Lapidus, R. J.; Gates, B. C. Metal clusters on supports: synthesis, structure, reactivity, and catalytic properties. Chem. Commun. 2010, 46, 5997-6015.
35. Meng, F.; Gong, Z.; Wang, Q.; et al. Effects of ZrO2 crystalline phase on oxygen vacancy of GaZr oxides and their properties for CO2 hydrogenation to light olefins. Catal. Today. 2024, 433, 114661.
36. Martínez Figueredo, K. G.; Martínez, F. A.; Segobia, D. J.; Bertero, N. M. Valeric biofuels from biomass-derived γ-valerolactone: a critical overview of production processes. Chempluschem 2023, 88, e202300381.
37. Yan, P.; Wang, H.; Liao, Y.; Wang, C. Zeolite catalysts for the valorization of biomass into platform compounds and biochemicals/biofuels: a review. Renew. Sustain. Energy. Rev. 2023, 178, 113219.
38. Victor, A.; Sharma, P.; Pulidindi, I. N.; Gedanken, A. Levulinic acid is a key strategic chemical from biomass. Catalysts 2022, 12, 909.
39. Hou, P.; Su, H.; Jin, K.; Li, Q.; Yan, W. Zirconium phosphate-pillared zeolite MCM-36 for green production of γ-valerolactone from levulinic acid via catalytic transfer hydrogenation. Molecules 2024, 29, 3779.
40. Wang, J.; Jaenicke, S.; Chuah, G. Zirconium–Beta zeolite as a robust catalyst for the transformation of levulinic acid to γ-valerolactone via Meerwein–Ponndorf–Verley reduction. RSC. Adv. 2014, 4, 13481-9.
41. Morales, G.; Melero, J. A.; Iglesias, J.; Paniagua, M.; López-Aguado, C. From levulinic acid biorefineries to γ-valerolactone (GVL) using a bi-functional Zr-Al-Beta catalyst. React. Chem. Eng. 2019, 4, 1834-43.
42. López-Aguado, C.; Paniagua, M.; Melero, J. A.; et al. Stable continuous production of γ-valerolactone from biomass-derived levulinic acid over Zr–Al-Beta zeolite catalyst. Catalysts 2020, 10, 678.
43. Antunes, M. M.; Silva, A. F.; Fernandes, A.; Pillinger, M.; Ribeiro, F.; Valente, A. A. Renewable bio-based routes to γ-valerolactone in the presence of hafnium nanocrystalline or hierarchical microcrystalline zeotype catalysts. J. Catal. 2022, 406, 56-71.
44. Gao, M.; Yang, G.; He, G.; et al. Integrating zeolite and layered double hydroxide for highly selective catalytic production of γ-valerolactone. CCS. Chem. 2024, 6, 652-62.
45. Zhang, B.; Wu, Q.; Zhang, C.; et al. A robust Ru/ZSM-5 hydrogenation catalyst: insights into the resistances to ruthenium aggregation and carbon deposition. ChemCatChem 2017, 9, 3646-54.
46. Popova, M.; Djinović, P.; Ristić, A.; et al. Vapor-phase hydrogenation of levulinic acid to γ-valerolactone over Bi-functional Ni/HZSM-5 catalyst. Front. Chem. 2018, 6, 285.
47. Wang, Y.; Bao, J.; Zuo, C.; et al. Ultra-small PtNi bimetallic encapsulated in silicalite-1 zeolite with fine-tuned surface acidity for selective conversion of levulinic acid. Appl. Organomet. Chem. 2023, 37, e6935.
48. Abusuek, D. A.; Tkachenko, O. P.; Bykov, A. V.; et al. ZSM-5 as a support for Ru-containing catalysts of levulinic acid hydrogenation: influence of the reaction conditions and the zeolite acidity. Catal. Today. 2023, 423, 113885.
49. Liang, W.; Xu, G.; Zhang, X.; Chen, H.; Fu, Y. MFI zeolite with confined adjustable synergistic Cu sites for the hydrogenation of levulinic acid. Green. Chem. 2024, 26, 498-506.
50. Li, J.; Li, D.; Yu, P.; et al. CeOx-induced oxygen vacancy-enhanced Pt-based titanium silicalite-1 catalysts for selective conversion of levulinic acid. Appl. Organomet. Chem. 2024, 38, e7447.
51. Varimalla, S.; Manda, K.; Boggala, S.; et al. Effect of method of preparation of Ni and/or Cu supported on ZSM-5 catalysts for the aqueous phase hydrogenation of levulinic acid to γ-valerolactone. Catal. Today. 2024, 441, 114916.
52. Yi, Z.; Hu, D.; Xu, H.; Wu, Z.; Zhang, M.; Yan, K. Metal regulating the highly selective synthesis of gamma-valerolactone and valeric biofuels from biomass-derived levulinic acid. Fuel 2020, 259, 116208.
53. Chen, X.; Deng, L.; Zhou, S.; Qiao, C.; Tian, Y. Alkaline modified Zr-loading on nanosheet zeolite towards production of γ-valerolactone from levulinic acid through catalytic transfer hydrogenation. Appl. Catal. A. Gen. 2024, 682, 119816.
54. Vu, H. T.; Harth, F. M.; Wilde, N. Silylated zeolites with enhanced hydrothermal stability for the aqueous-phase hydrogenation of levulinic acid to γ-valerolactone. Front. Chem. 2018, 6, 143.
55. Vu, H. T.; Goepel, M.; Gläser, R. Improving the hydrothermal stability of zeolite Y by La3+ cation exchange as a catalyst for the aqueous-phase hydrogenation of levulinic acid. RSC. Adv. 2021, 11, 5568-79.
56. Hu, D.; Xu, H.; Wu, Z.; et al. Noble metal-free hierarchical ZrY zeolite efficient for hydrogenation of biomass-derived levulinic acid. Front. Chem. 2021, 9, 725175.
57. Vu, H.; Harth, F. M.; Goepel, M.; Linares, N.; García–Martínez, J.; Gläser, R. Enhanced activity of a bifunctional Pt/zeolite Y catalyst with an intracrystalline hierarchical pore system in the aqueous-phase hydrogenation of levulinic acid. Chem. Eng. J. 2022, 430, 132763.
58. Jayakumari, M. T.; Kanakkampalayam Krishnan, C. Tuning Al sites in Y-zeolite for selective production of γ-valerolactone from levulinic acid. Appl. Catal. A. Gen. 2023, 663, 119318.
59. Al-Khawlani, A.; Wang, Y.; Bao, J.; et al. Enhanced catalytic activity and high stability of treated Pt-Ru/ zeolite Y catalysts for levulinic acid hydrogenation reaction. Catal. Commun. 2023, 183, 106761.
60. Wang, Y.; Zhou, Y.; Bao, J.; et al. Molecular synergistic synthesis of AIPO-18 zeolite-stabilized Pt nanocatalysts with high dispersion for the hydrogenation of levulinic acid to γ-valerolactone. Appl. Organomet. Chem. 2022, 36, e6646.
61. Al-Khawlani, A.; Bao, J.; Sheng, X.; et al. SSZ-39 zeolite-based Ru catalysts for selective hydrogenation of levulinic acid to γ-valerolactone: influence of synthesis method and zeolite acidity. Micropor. Mesopor. Mat. 2024, 372, 113112.
62. Li, W.; Li, F.; Chen, J.; et al. Efficient and sustainable hydrogenation of levulinic acid to γ-valerolactone in aqueous phase over Ru/MCM-49 catalysts. Ind. Eng. Chem. Res. 2020, 59, 17338-47.
63. Li, W.; Li, F.; Ning, X.; et al. Promotional effect of Mn doping on Ru/layered MCM-49 catalysts for the conversion of levulinic acid to γ-valerolactone. Carbon. Resour. Convers. 2022, 5, 185-92.
64. Dutta, S.; Yu, I. K.; Tsang, D. C.; et al. Green synthesis of gamma-valerolactone (GVL) through hydrogenation of biomass-derived levulinic acid using non-noble metal catalysts: a critical review. Chem. Eng. J. 2019, 372, 992-1006.
65. Boronat, M.; Concepcion, P.; Corma, A.; Renz, M.; Valencia, S. Determination of the catalytically active oxidation Lewis acid sites in Sn-beta zeolites, and their optimisation by the combination of theoretical and experimental studies. J. Catal. 2005, 234, 111-8.
66. Lee, A.; Chaibakhsh, N.; Rahman, M. B. A.; Basri, M.; Tejo, B. A. Optimized enzymatic synthesis of levulinate ester in solvent-free system. Ind. Crops. Prod. 2010, 32, 246-51.
67. Joshi, H.; Moser, B. R.; Toler, J.; Smith, W. F.; Walker, T. Ethyl levulinate: a potential bio-based diluent for biodiesel which improves cold flow properties. Biomass. Bioenergy. 2011, 35, 3262-6.
68. Lu, T.; You, X.; Zong, Y.; Xu, Y.; Yang, X.; Zhou, L. Production of γ-valerolactone from ethyl levulinate over hydrothermally synthesized Sn-Beta under mild conditions. Fuel 2023, 332, 126262.
69. Qu, H.; Lu, T.; Yang, X.; Zhou, L. Promoting tin into the framework of β zeolite via stabilizing Sn species and its catalytic performance for the conversion of ethyl levulinate to γ-valerolactone. Renew. Energy. 2024, 229, 120746.
70. Zhang, Z.; Liu, Z.; Gu, Z.; Wen, Z.; Xue, B. Selective production of γ-valerolactone from ethyl levulinate by catalytic transfer hydrogenation over Zr-based catalyst. Res. Chem. Intermed. 2022, 48, 1181-98.
71. Tang, B.; Li, S.; Song, W.; et al. Hierarchical FAU-type hafnosilicate zeolite as a robust lewis acid catalyst for catalytic transfer hydrogenation. ACS. Sustain. Chem. Eng. 2019, 7, 16329-43.
72. Chen, C.; Chen, M.; Zada, B.; et al. Effective conversion of biomass-derived ethyl levulinate into γ-valerolactone over commercial zeolite supported Pt catalysts. RSC. Adv. 2016, 6, 112477-85.
73. Zhang, L.; Xi, G.; Yu, K.; Yu, H.; Wang, X. Furfural production from biomass–derived carbohydrates and lignocellulosic residues via heterogeneous acid catalysts. Ind. Crops. Prod. 2017, 98, 68-75.
74. Antunes, M. M.; Lima, S.; Neves, P.; et al. One-pot conversion of furfural to useful bio-products in the presence of a Sn,Al-containing zeolite beta catalyst prepared via post-synthesis routes. J. Catal. 2015, 329, 522-37.
75. Wang, Q.; Qi, W.; Wang, W.; et al. Production of furfural with high yields from corncob under extremely low water/solid ratios. Renew. Energy. 2019, 144, 139-46.
76. Kong, X.; Zhang, X.; Han, C.; Li, C.; Yu, L.; Liu, J. Ethanolysis of biomass based furfuryl alcohol to ethyl levulinate over Fe modified USY catalyst. Mol. Catal. 2017, 443, 186-92.
77. Song, D.; An, S.; Lu, B.; Guo, Y.; Leng, J. Arylsulfonic acid functionalized hollow mesoporous carbon spheres for efficient conversion of levulinic acid or furfuryl alcohol to ethyl levulinate. Appl. Catal. B. Environ. 2015, 179, 445-57.
78. Wang, R.; Wang, J.; Zi, H.; Xia, Y.; Wang, H.; Liu, X. Catalytic transfer hydrogenation of ethyl levulinate to γ-valerolactone over zirconium (IV) Schiff base complexes on mesoporous silica with isopropanol as hydrogen source. Mol. Catal. 2017, 441, 168-78.
79. Melero, J. A.; Morales, G.; Iglesias, J.; et al. Efficient one-pot production of γ-valerolactone from xylose over Zr-Al-Beta zeolite: rational optimization of catalyst synthesis and reaction conditions. Green. Chem. 2017, 19, 5114-21.
80. Hernández, B.; Iglesias, J.; Morales, G.; et al. One-pot cascade transformation of xylose into γ-valerolactone (GVL) over bifunctional Brønsted–Lewis Zr–Al-beta zeolite. Green. Chem. 2016, 18, 5777-81.
81. Li, X.; Yuan, X.; Xia, G.; et al. Catalytic production of γ-valerolactone from xylose over delaminated Zr-Al-SCM-1 zeolite via a cascade process. J. Catal. 2020, 392, 175-85.
82. López-Aguado, C.; Paniagua, M.; Iglesias, J.; Morales, G.; García-Fierro, J. L.; Melero, J. A. Zr-USY zeolite: efficient catalyst for the transformation of xylose into bio-products. Catal. Today. 2018, 304, 80-8.
83. Sun, W.; Li, H.; Wang, X.; Liu, A. Cascade upgrading of biomass-derived furfural to γ-valerolactone over Zr/Hf-based catalysts. Front. Chem. 2022, 10, 863674.
84. Winoto, H. P.; Ahn, B. S.; Jae, J. Production of γ-valerolactone from furfural by a single-step process using Sn-Al-Beta zeolites: optimizing the catalyst acid properties and process conditions. J. Ind. Eng. Chem. 2016, 40, 62-71.
85. Song, S.; Di, L.; Wu, G.; Dai, W.; Guan, N.; Li, L. Meso-Zr-Al-beta zeolite as a robust catalyst for cascade reactions in biomass valorization. Appl. Catal. B. Environ. 2017, 205, 393-403.
86. Melero, J. A.; Morales, G.; Iglesias, J.; Paniagua, M.; López-Aguado, C. Rational optimization of reaction conditions for the one-pot transformation of furfural to γ-valerolactone over Zr–Al-beta zeolite: toward the efficient utilization of biomass. Ind. Eng. Chem. Res. 2018, 57, 11592-9.
87. Winoto, H. P.; Fikri, Z. A.; Ha, J.; et al. Heteropolyacid supported on Zr-Beta zeolite as an active catalyst for one-pot transformation of furfural to γ-valerolactone. Appl. Catal. B. Environ. 2019, 241, 588-97.
88. Srinivasa Rao, B.; Krishna Kumari, P.; Koley, P.; Tardio, J.; Lingaiah, N. One pot selective conversion of furfural to γ-valerolactone over zirconia containing heteropoly tungstate supported on β-zeolite catalyst. Mol. Catal. 2019, 466, 52-9.
89. Barakov, R.; Shcherban, N.; Petrov, O.; et al. Optimization of Zr-Al-USY and Zr-Al-Beta zeolites catalysts for a one-pot cascade transformation of furfural to γ-valerolactone. Catal. Today. 2024, 426, 114406.
90. Liu, Z.; Zhang, R.; Liu, H.; et al. Effect of carbon modifier on characteristics and catalytic properties of zeolite–carbon hybrid supported Zr towards γ-valerolactone production. Fuel 2024, 359, 130380.
91. Qiu, J.; Liu, Y.; Zhang, J.; et al. One-pot cascade process for efficient upgrading of furfural to γ-valerolactone over adjustable Lewis-Brønsted bi-acidic catalyst. Ind. Crops. Prod. 2024, 214, 118474.
92. Zhu, S.; Xue, Y.; Guo, J.; Cen, Y.; Wang, J.; Fan, W. Integrated conversion of hemicellulose and furfural into γ-valerolactone over Au/ZrO2 catalyst combined with ZSM-5. ACS. Catal. 2016, 6, 2035-42.
93. Kim, K. D.; Kim, J.; Teoh, W. Y.; Kim, J. C.; Huang, J.; Ryoo, R. Cascade reaction engineering on zirconia-supported mesoporous MFI zeolites with tunable Lewis-Brønsted acid sites: a case of the one-pot conversion of furfural to γ-valerolactone. RSC. Adv. 2020, 10, 35318-28.
94. Shao, Y.; Guo, M.; Fan, M.; et al. Alloying nickel and cobalt with iron on ZSM-5 for tuning competitive hydrogenation reactions for selective one-pot conversion of furfural to gamma-valerolactone. Dalton. Trans. 2022, 51, 17441-53.
95. Tolek, W.; Auppahad, W.; Weerachawanasak, P.; Mekasuwandumrong, O.; Praserthdam, P.; Panpranot, J. One-pot conversion of furfural to γ-valerolactone over Co- and Pt-doped ZSM-5 catalysts. Catalysts 2023, 13, 498.
96. Liu, B.; Chen, X.; Xu, Y.; Qiao, C.; Lu, Z.; Tian, Y. A combo Zr–zeolite and Zr(OH)4 mixture composition for one–pot production of γ–valerolactone from furfural. Renew. Energy. 2024, 229, 120751.
97. Zhang, H.; Yang, W.; Roslan, I. I.; Jaenicke, S.; Chuah, G. A combo Zr-HY and Al-HY zeolite catalysts for the one-pot cascade transformation of biomass-derived furfural to γ-valerolactone. J. Catal. 2019, 375, 56-67.
98. Tang, B.; Li, S.; Song, W.; Li, Y.; Yang, E. One-pot transformation of furfural into γ-valerolactone catalyzed by a hierarchical Hf-Al-USY zeolite with balanced Lewis and Brønsted acid sites. Sustain. Energy. Fuels. 2021, 5, 4724-35.
99. García, A.; Sánchez-Tovar, R.; Miguel, P. J.; et al. Catalytic production of γ-valerolactone, a biofuel precursor, from furfural in one-pot: synergistic effect between Zr and Sn. Fuel 2023, 352, 129045.
100. Jayakumari, M. T.; Krishnan, C. K. Modulating acid sites in Y zeolite for valorisation of furfural to get γ-valerolactone. RSC. Adv. 2024, 14, 21453-63.
101. Bui, L.; Luo, H.; Gunther, W. R.; Román-Leshkov, Y. Domino reaction catalyzed by zeolites with Brønsted and Lewis acid sites for the production of γ-valerolactone from furfural. Angew. Chem. Int. Ed. Engl. 2013, 52, 8022-5.
102. Gao, X.; Yu, X.; Peng, L.; He, L.; Zhang, J. Magnetic Fe3O4 nanoparticles and ZrO2-doped mesoporous MCM-41 as a monolithic multifunctional catalyst for γ-valerolactone production directly from furfural. Fuel 2021, 300, 120996.
103. Yan, K.; Wu, G.; Lafleur, T.; Jarvis, C. Production, properties and catalytic hydrogenation of furfural to fuel additives and value-added chemicals. Renew. Sustain. Energy. Rev. 2014, 38, 663-76.
104. Wang, H.; Ding, G.; Liu, Y.; Zhang, J.; Li, Y.; Zhu, Y. Highly effective production of levulinic acid and γ-valerolactone through self-circulation of solvent in a continuous process. React. Chem. Eng. 2021, 6, 1811-8.
105. Lima, T. M.; Lima, C. G. S.; Rathi, A. K.; et al. Magnetic ZSM-5 zeolite: a selective catalyst for the valorization of furfuryl alcohol to γ-valerolactone, alkyl levulinates or levulinic acid. Green. Chem. 2016, 18, 5586-93.
106. Karanwal, N.; Kurniawan, R. G.; Park, J.; et al. One-pot, cascade conversion of cellulose to γ-valerolactone over a multifunctional Ru–Cu/zeolite-Y catalyst in supercritical methanol. Appl. Catal. B. Environ. 2022, 314, 121466.
107. Lin, Q. F.; Gao, Z. R.; Lin, C.; et al. A stable aluminosilicate zeolite with intersecting three-dimensional extra-large pores. Science 2021, 374, 1605-8.
108. Paniagua, M.; Morales, G.; Melero, J. A.; et al. Understanding the role of Al/Zr ratio in Zr-Al-Beta zeolite: towards the one-pot production of GVL from glucose. Catal. Today. 2021, 367, 228-38.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Data & Comments
Data


















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.