fig2
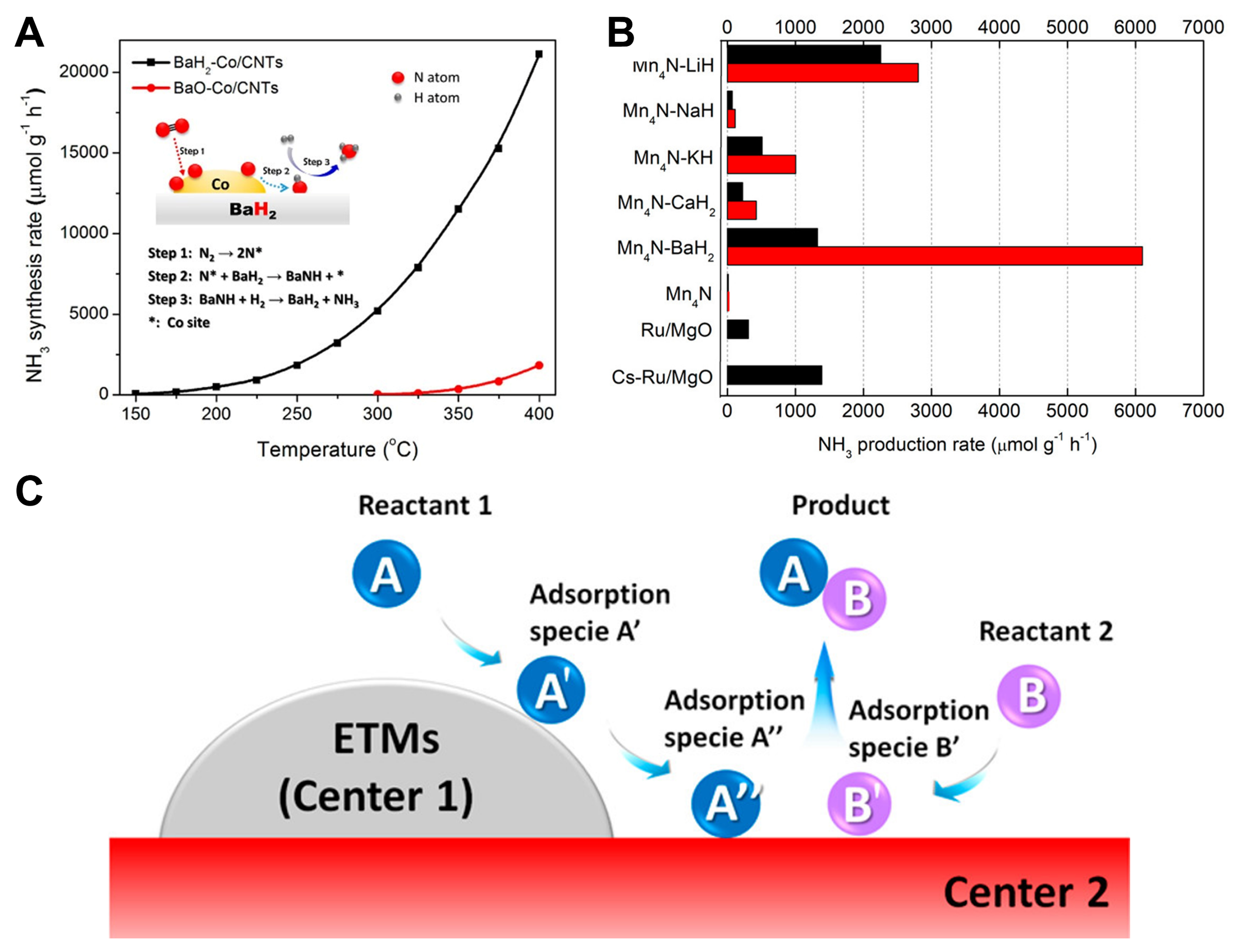
Figure 2. (A) Temperature dependence of the NH3 synthesis rates of BaH2-Co/CNTs and BaO-Co/CNTs. The insert of (A) shows the three steps of NH3 synthesis catalyzed by BaH2-Co/CNTs. Reproduced from Ref[30]. Copyright (2017), with permission from American Chemical Society; (B) Comparison of NH3 yield of different catalysts, reaction condition: 10 bar syn-gas (N2:H2 = 1:3), 573 K,








