A systematic review of renal mass biopsy: what evidence supports its use (or omission)?
Abstract
Aim: Management of clinically localized renal masses ≤ 7 cm (cT1RM) is typically guided by imaging rather than renal mass biopsy (RMB), unlike most other malignancies, where tissue diagnosis precedes treatment decisions. Despite advancements, fewer than 25% of cT1RM patients undergo RMB before surgery or active surveillance (AS). While imaging modalities such as CT, magnetic resonance imaging (MRI), and ultrasound can often identify benign lesions (e.g., Bosniak I-IIF cysts, angiomyolipoma), decisions regarding solid, enhancing masses and Bosniak III-IV cysts frequently proceed without histological confirmation of renal cell carcinoma. This practice may lead to potentially unnecessary or “needless” interventions. AS has emerged as a viable alternative to surgery for renal masses < 3-4 cm, showing low metastatic progression rates comparable to those seen with intervention.
Methods: We systematically reviewed observational studies (retrospective and prospective) due to the absence of randomized controlled trials on RMB for cT1RM.
Results: Reported sensitivity, specificity, positive predictive value, and negative predictive value of RMB range from 93%-99%, 71%-100%, 97%-100%, and 38%-63%, respectively. Safety data affirm RMB as a low-risk procedure, with minor complications (e.g., bleeding, hematoma) occurring in < 5% of cases and hospital admissions/readmissions in < 3%.
Conclusion: While RMB is accurate, its role in treatment planning remains underexplored. Emerging evidence suggests increased adoption of non-interventional approaches (e.g., AS), with RMB influencing treatment decisions (e.g., avoidance of nephrectomy) in 10%-30% of cases. Future studies should investigate when RMB is warranted, its influence on treatment selection, and its effects on patient-centered outcomes such as decisional conflict and regret.
Keywords
INTRODUCTION
Renal cell carcinoma (RCC) is the third most common malignancy affecting the urinary system and represents a heterogeneous collection of malignancies that accounts for approximately 90% of adult kidney cancers worldwide[1]. Over the past few decades, the detection of small renal masses (SRMs) - defined as tumors measuring ≤ 4 cm (cT1aRM) - has steadily increased, primarily due to the widespread use of cross-sectional computed tomography (CT) and magnetic resonance imaging (MRI). A significant proportion of these SRMs are localized and have low metastatic potential; they are frequently cured with kidney-sparing interventions (KSIs)[2,3]. Increasing evidence supports the role of non-interventional management, such as active surveillance (AS), for cT1aRM with similarly low rates of progression to metastasis or cancer-related death in patients selected for these approaches[4-6]. Immediate intervention is generally selected for renal masses (RMs) > 7 cm because of the increased likelihood that such RMs harbor potentially aggressive malignancies[7,8]. For clinically localized RMs measuring > 4 and ≤ 7 cm in size (cT1bRM), there are several treatment options, ranging from radical nephrectomy (RN) to AS. Variability in the selection of treatment has been observed, with partial nephrectomy (PN) offered at rates between 0% and 90% for cT1bRM (data not shown)[9-11]. There is increasing evidence that select patients with cT1bRM can be managed safely with non-interventional approaches, such as AS for RCC and expectant management[12] (or less AS), or reassurance if benign features are identified on imaging and/or pathology (manuscript under revision). Thus, the optimal management of T1 renal masses (cT1RM) remains an area of active debate[4,13]. In past decades, RN was considered the standard of care for localized renal lesions. However, advances in nephron-sparing surgery, minimally invasive surgery, ablative techniques (such as cryoablation, radiofrequency, and microwave ablation), radiation therapy [stereotactic body radiation therapy (SBRT)], and AS protocols have expanded the available therapeutic options.
The 2016 World Health Organization (WHO) classification of RCC is based on features such as cytoplasmic (clear cell RCC and chromophobe RCC), architectural (papillary RCC), and anatomic location (collecting duct and renal medullary carcinomas)[14]. The most common subtypes are clear cell RCC, which accounts for 70%-75% of all RCCs, followed by papillary RCC (10%-21%) and chromophobe RCC (~5%)[15]. The 2022 WHO Classification of Tumors Part A provided several updates, including oncocytic and chromophobe renal tumors as a part of the four major categories: clear cell renal tumors, papillary renal cell tumors, oncocytic and chromophobe renal tumors, and collecting duct tumors[16].
Despite growing interest in nephron-sparing treatments because of its renal functional advantages, there is a lack of consensus regarding (1) when to opt for surgery; (2) if surgery is indicated, whether to perform robotic partial nephrectomy (RPN), open PN (OPN), or minimally-invasive RN (MIRN); (3) if surgery poses increased risk and the patient should undergo treatment, whether to elect SBRT or an ablative technique, and which ablative modality is most appropriate; (4) and how to identify patients who might benefit most from AS. Decision making is further complicated by factors such as comorbidities, patient preferences, and tumor characteristics (e.g., size, complexity, features suggesting higher-risk cancer and/or local invasiveness, and growth rate during AS). In response, various organizations have developed clinical practice guidelines. Nevertheless, the care patients receive often differs substantially depending on the treating provider, institutional practices, and regional healthcare systems[17].
History and timeline of renal mass biopsy
In the first half of the 20th century, RMs were typically identified only after the onset of symptoms. RCC, known as the “internist’s tumor”, classically presented with the triad of a palpable abdominal mass, flank pain, and hematuria. However, with the increasing use of CT, most RMs began to be detected incidentally - transforming RCC into the “radiologist’s tumor”. These cT1RMs were most often small and asymptomatic[18]. The resolution and precision of CT, ultrasound, and MRI have improved markedly over time[19]. Advanced radiographic techniques, either performed during scanning or through post-scan processing, can help distinguish certain RCC subtypes from others[20,21]. Nonetheless, imaging alone continues to face limitations in distinguishing between RCC subtypes. Approximately 25% of RMs measuring < 4 cm are ultimately benign. As of 2025, no imaging technique is sufficiently reliable to define the histologic subtype of RCC. By contrast, renal mass biopsy (RMB) achieves > 90% accuracy in determining the histological type of renal cancer[22].
In the late 20th century, RMB was not routinely performed for several reasons. First, the diagnostic performance of available percutaneous techniques was considered inadequate. Fine-needle aspiration (FNA) and core biopsy without coaxial equipment often failed to yield reliable samples. Even more concerning was the potential risk of tumor seeding associated with non-coaxial approaches. A study by Ali et al. reported that only 8% of urologists performed RMB in 20% of their SRM patients, while nearly 73% had never performed RMB in this population[23]. By the turn of the century, however, some urologists began to reconsider the dismissal of RMB[24]. Although concerns such as low negative predictive value (NPV), sampling error, tumor heterogeneity, and difficulty in differentiating tumor types persisted, the overall utility of RMB, particularly when it provided a definitive diagnosis of benign neoplasms, was increasingly recognized[24]. Since 2000, numerous studies on RMB have been published, most focusing on technical aspects and pathologic outcomes in patients undergoing RMB[25,26].
Practical considerations: how is RMB performed?
The approach to RMB has evolved significantly since its transition from open surgical techniques to needle-based methods in 1951[27]. Tumor targeting and needle placement are typically guided by CT or ultrasound, depending on patient and tumor characteristics[28]. FNA employs 21-gauge or smaller needles to collect multiple samples for cytological analysis. In contrast, core biopsy utilizes larger 18-gauge needles that retrieve intact tissue within the needle lumen, thereby, reducing the risk of contact between sampled tumor tissue and surrounding tissues during needle extration. A minimum of two non-fragmented core samples (from both central and peripheral tumor regions, each > 10 mm in length) should be sent for histological examination[29]. Without a coaxial sheath, multiple passes are required to obtain multiple cores. A coaxial sheath, however, enables multiple passes from within the same entry site while providing an additional barrier between the body and potentially cancerous tissue, thereby, lowering the risk of tumor seeding[30,31]. Although early reports of severe outcomes led to long-standing concerns - based on only a few cases involving aggressive non-RCC malignancies and one case of papillary RCC[32,33] - the current incidence of tumor seeding with contemporary techniques is approximately 1:10.000[30,34].
In the United States, most RMB procedures are performed in hospitals by interventional radiologists, typically with same-day discharge and optional overnight observation[25]. Early reports demonstrated the feasibility of outpatient, ultrasound-guided RMB performed by urologists in office settings, with low complication rates[35,36]. Rasmussen et al. reported an overall complication rate of 3.8% for outpatient RMB, with 1% classified as major and 2.8% as minor[37]. More recent studies have confirmed the safety and efficacy of office-based RMB performed by urologists[38-40]. In contrast, a survey conducted by the American Urological Association (AUA) found that urologists independently perform only about 2% of RMB procedures, with most relying on interventional radiology support[41]. A recent cost analysis by Patel et al. compared office-based vs. hospital-based RMB, finding the mean cost of an office-based percutaneous RMB to be $2,129, compared to $4,598 in a hospital setting[39].
Biopsy: why or why not?
There are limited data directly explaining why urologists refrain from using RMB to evaluate newly detected SRMs. However, many consider RMB to have limited clinical significance in influencing treatment decisions. In a survey by Khan et al. (2007), 43% (139 of 325) of urologists reported never using biopsy[42]. Among those, 87% cited concerns about false-negative results, and 58% did not believe biopsy findings would alter patient management[42]. Less commonly cited reasons included the risk of tumor seeding, biopsy-related complications, histopathological uncertainty, false-positive results, and lack of access to a uro-radiologist[42]. Similarly, Lim et al. reported that some urologists avoided RMB when their institution lacked interventional radiologists experienced in performing the procedure, citing concerns about false-positive results[2].
How successful is RMB as a technique?
When using core biopsy rather than FNA, RMB provides an informative result in 82-95% of cases[25,26]. Indeterminate (nondiagnostic) outcomes occur in 8%-14% of cases, usually due to inadequate sampling or the collection of normal kidney tissue or tissue from a non-kidney source. A mismatch between imaging abnormalities and pathology results does not necessarily rule out cancer. Indeterminate results can be managed with repeat RMB, alternate imaging, or clinical decision making under uncertainty. Repeat RMB has been shown to reduce nondiagnostic outcomes and provide actionable histological findings in over 95% of cases[22]. After RMB, four possible outcomes exist: (1) nondiagnostic; (2) confirmed accurate - validated by subsequent surgery or repeat biopsy; (3) presumed accurate - without further pathology; or (4) proved inaccurate - demonstrated by discordant surgical pathology results. Although this classification best reflects RMB performance, most studies focus on operating characteristics, with relatively few reporting accuracy or these outcome categories.
When adequate tissue is obtained, systematic reviews report sensitivity of 95.2%-99% and specificity of 60%-100% for cancer diagnosis [Table 1]. The false-positive rate is estimated at 0%-5%, depending on pretest probability[24]. The main limitation of RMB is its false-negative rate, which ranges from 0%-25% when considered in isolation[24]. For example, a RMB suggesting a benign renal neoplasm such as an oncocytoma may later be found at nephrectomy to represent chromophobe RCC or another oncocytic RCC - a false negative. Conversely, an oncocytic neoplasm suspicious for RCC on biopsy may prove to be benign after resection - a false positive. To address this, the 2022 WHO guidelines recommend the term “oncocytic renal neoplasm, not further classified” rather than oncocytoma in RMB reporting, acknowledging limitations in sampling and overlap with malignant tumors[16]. Several experts have emphasized the importance of interpreting “benign” RMB findings with caution, as they may still conceal malignancy[43,44]. For such patients, we recommend AS rather than simple reassurance, with imaging at 6 months and annually for 2-3 years, reserving repeat RMB or intervention for cases with accelerated growth.
Diagnostic accuracy metrics from systematic reviews and retrospective studies on RMB
| N | Nondiagnostic rate | Sensitivity | Specificity | PPV | NPV | |
| Systematic reviews | ||||||
| Patel et al., 2016[26] | 2,979 | 14.1% | 97.5% | 96.2% | 99.8% | 63.3% |
| Marconi et al., 2016[25] | 5,228 | 0%-22.6% | 99.1% | 99.7% | NR | NR |
| Retrospective studies (2016-present) | ||||||
| Londoño et al., 2013[87] | 126 | 5% (7) | 75.4% | 100% | 100% | 11.7% |
| Bernhard et al., 2015[88] | 130 | 7.7% (10) | NR | NR | NR | NR |
| Garnon et al., 2015[89] | 26 | 0% (0) | 95.4% | 100% | 100% | 75% |
| Prince et al., 2015[90] | 565 | 14.7% (83) | NR | NR | NR | NR |
| Jeon et al., 2016[91] | 442 | 11.1% (49) | NR | NR | NR | NR |
| Richard et al., 2015[64] | 509 | 10% (53) | NR | NR | NR | NR |
| Dave et al., 2017[36] | 108 | 13% (14) | NR | NR | NR | NR |
| Alle et al., 2018[92] | 184 | 11.4% (21) | NR | NR | NR | NR |
| Wells et al., 2017[75] | 213 | 1.4% (3)a | NR | NR | NR | NR |
| Tsang Mui Chung et al., 2018[93] | 317 | 6% (19) | NR | NR | NR | NR |
| Posielski et al., 2019[94] | 965 | 15% (145) | NR | NR | NR | NR |
| Gillis et al., 2020[95] | 423 | 16% (66) | NR | NR | NR | NR |
| Ma et al., 2020[66] | 174 | 9% (15) | NR | NR | NR | NR |
| Santy et al., 2020[96] | 180 | 12.8% (23) | NR | NR | NR | NR |
| Shahait et al., 2020[97] | 175 | 9% (16) | 89% | 71% | 97% | 38% |
| Bada et al., 2021[98] | 94 | 6.4% (6) | NR | NR | NR | NR |
| Ferrari et al., 2021[99] | 78 | 20.5 (16) | NR | NR | NR | NR |
| Masic et al., 2021[100] | 306 | 8.2% (25) | 93% | 93% | 99% | 52% |
| Takafuji et al., 2021[76] | 234 | 9.4% (22) | NR | NR | NR | NR |
| Jiang et al., 2022[38] | 121 | 17% (33) | NR | NR | NR | NR |
| Jasinski et al., 2022[101] | 247 | 18.6% (46) | NR | NR | NR | NR |
| Kapur et al., 2022[102] | 805 | 8.0% (70) | NR | NR | NR | NR |
| Nazzani et al., 2022[103] | 102 | 1% (3) | NR | NR | NR | NR |
| Valtersson et al., 2022[104] | 484 | 16% (78) | NR | NR | NR | NR |
| Chen et al., 2024[67] | 158 | 14.6% (23) | NR | NR | NR | NR |
| Serhal et al., 2024[105] | 167 | 12% (20) | NR | NR | NR | NR |
| Median % (range) | 10.6% (0%-20.5%) | |||||
The accuracy of RMB for histologic subtyping of renal tumors ranges from 74%-100% in published reports[22,25,26]. Imaging alone is insufficient to classify renal tumor subtypes, highlighting the need for RMB in further assessment. RMB also contributes to evaluating tumor aggressiveness, which is essential for guiding treatment decisions. The Fuhrman nuclear grading system is strongly associated with oncologic outcomes[45]: grade 1 tumors exhibit a 0% metastatic rate, whereas up to 50% of higher-grade RCCs demonstrate metastatic potential[46]. Nuclear grading is the least reliable aspect of RMB due to tumor heterogeneity, with discordant grades between RMB and surgical specimens reported in up to 70% of cases[47,48]. However, accuracy improves when grade differences are within one tier of the final grade or when categorizing tumors as low-grade (1-2) versus high-grade (3-4). In a retrospective study, RMB showed 75% accuracy in determining RCC histologic subtype, rising to 93% when grouped into low- versus high-grade categories[49]. Similarly, Blumenfeld et al. reported accurate subtype identification in 71 of 81 cases (88%)[50]. While RMB has demonstrated strong accuracy for classification and grading, further refinement of biopsy techniques is needed to enhance diagnostic consistency.
Removal of benign tumors - potentially avoidable surgery
The surgical removal of benign renal masses has increased markedly in the 21st century. A systematic review reported an 82% rise in procedures (from 3,098 to 5,624 cases) during 2000-2009, with 20% of cT1aRM identified as benign during this period[51]. This represents approximately 6,000 patients undergoing potentially avoidable surgery, along with the associated financial burdens - an outcome that could likely have been prevented through the judicious use of RMB[51]. Kim et al. further reported that the overall prevalence of benign pathologic findings in PN exceeded 30% between 2007 and 2014[52]. The rising incidence of RCC has paralleled an increase in surgically treated RMs over time. Although surgery remains the cornerstone of RCC management, the persistently stable kidney cancer mortality rates raise concerns about potential overdiagnosis and overtreatment. RMB offers a critical alternative by enabling patients to avoid unnecessary aggressive and invasive surgical interventions.
Considering the diverse treatment modalities available for cT1RM, varying guideline recommendations, and the absence of definitive guidance on the appropriate use of RMB, a systematic review synthesizing the current evidence is essential. This review aims to examine published guidelines, randomized controlled trials, observational studies, and other high-quality evidence to establish best practices for RMB in the management of cT1RM. Specifically, it will address RMB utilization rates before treatment selection, its impact on treatment decision making, and both diagnostic and clinical outcomes. By consolidating the available evidence, this review seeks to better support clinicians in making informed treatment decisions for patients with cT1RM.
Objectives
The primary objective of this study is to assess the current evidence on the utilization and clinical value of RMB in patients with cT1RM. Specifically, it aims to evaluate how RMB informs treatment decision making, particularly with respect to selecting initial non-interventional strategies such as AS with the possibility of delayed intervention, rather than immediate intervention.
METHODS
Literature review
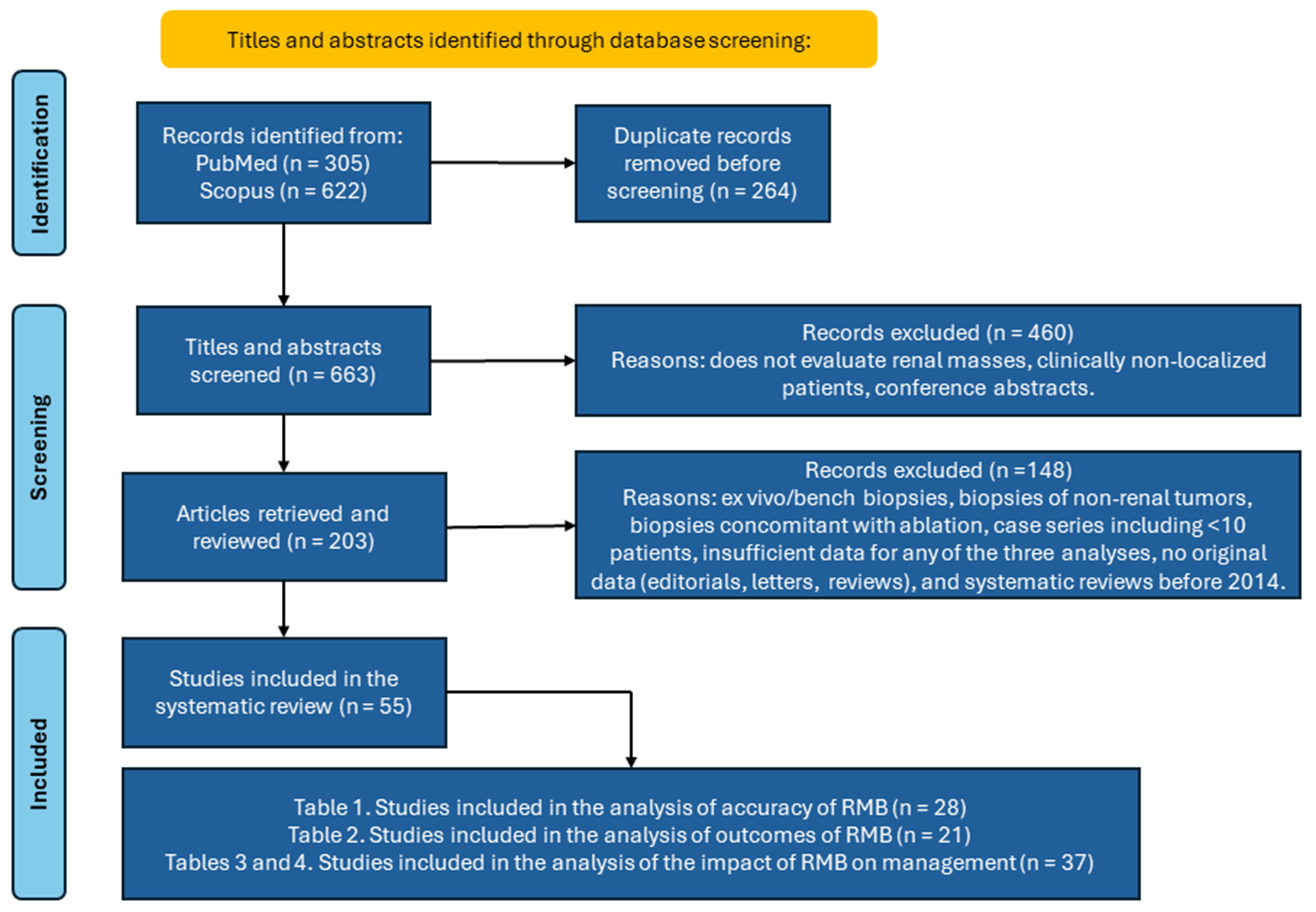
We searched PubMed and Scopus for English-language articles published between January 1969 and January 2025 using the terms: “renal mass biopsy” OR “renal tumor biopsy” OR “renal lesion biopsy” OR “kidney mass biopsy” OR “kidney tumor biopsy” OR “kidney lesion biopsy”. The search yielded 927 records (305 from PubMed and 622 from Scopus). After removing 264 duplicates, 663 articles were screened independently by two reviewers (I.O. and B.R.L.). At title/abstract screening, studies that did not evaluate renal masses, studies that enrolled patients with non-localized disease, and conference abstracts were excluded (n = 460). Following full-text review, an additional 148 records were excluded because they were case series with fewer than 10 patients; lacked original or sufficient data (editorials, letters, reviews); were systematic reviews published before 2014; or reported biopsies not applicable to this analysis (ex vivo/bench biopsies, non-renal tumor biopsies, or biopsies performed concomitantly with ablation). Fifty-five articles remained and were independently assessed by three researchers (I.O., M.S., and B.R.L.) and included in the systematic review [Figure 1]. Across the four manuscript tables, 86 studies are cited in total; 25 of these were referenced more than once in sub-analyses.
Inclusion and exclusion criteria
Eligibility criteria were specified to identify studies that examined the impact of RMB on treatment selection for masses suspicious for RCC. We included original studies focused on cT1RM and excluded studies of locally advanced or metastatic disease. Studies of pediatric populations (< 18 years) were excluded. Original articles reporting technical, post-procedural, and/or oncologic outcomes were eligible. We also included studies discussing the operating characteristics of RMB, indications for RMB, and complications associated with RMB. These criteria were intended to ensure that included studies provided robust, relevant data on the effectiveness and outcomes of RMB for cT1RM.
Study appraisal and synthesis
The review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Screening used a two-stage process: an initial title/abstract screen against predetermined inclusion and exclusion criteria, followed by a detailed full-text review of potentially eligible articles [Figure 1].
RESULTS
From 927 unique citations, we identified 55 studies related to RMB that met the inclusion criteria. Figure 1 shows the study selection process using the PRISMA flowchart. This systematic review addresses three main questions: (1) the diagnostic accuracy of RMB; (2) the clinical outcomes of RMB; and (3) the impact of RMB on initial treatment selection.
Diagnostic accuracy of RMB
Several operating characteristics of RMB have been examined in prior literature, including sensitivity, specificity, false-positive rate, false-negative rate, positive predictive value (PPV), and NPV [Table 1]. An important parameter is the nondiagnostic rate, which reflects whether adequate tissue has been collected for the pathologist to determine the nature of the mass. Pathologists classify the tissue as consistent with renal neoplasm, renal parenchyma, or non-renal tissue[4]. When RMB yields a definitive pathological diagnosis - whether malignant or benign - it can guide treatment decisions and potentially prevent unnecessary surgical interventions. Table 1 summarizes prior literature, including two well-conducted systematic reviews published in 2016[25,26], as well as articles published subsequently. Reported nondiagnostic rates for RMB range from 0% to 26%, reflecting variability across studies[25]. The two systematic reviews reported a median diagnostic rate of 86% for RMB, with a nondiagnostic rate of 14%. In studies published after those reviews, the median nondiagnostic rate was 10.6% (range: 0%-20.5%). One study reported a diagnostic rate of 86.9% for core needle biopsies of SRMs, with benign neoplasms accounting for 14.3% of cases[53].
Across the systematic reviews, the operating characteristics of RMB were as follows: sensitivity 96.7%-99.7%, specificity 94.4%-99%, PPV 96%-99.8%, and NPV 63%-86%. In 26 more recent publications, the reported ranges were sensitivity 75.4%-95.4%, specificity 71%-100%, PPV 97%-100%, and NPV 11.7%-75%. These results are broadly consistent with prior reviews, except for one study reporting a markedly lower NPV. This issue is further discussed in the section “How successful is RMB as a technique?”. Overall, our findings update and confirm the diagnostic performance of RMB reported in earlier systematic reviews.
Clinical outcomes of RMB
Although RMB is most commonly performed as an outpatient procedure, some patients experience adverse events, including immediate complications that require prolonged observation or intervention, overnight hospitalization, and/or return visits for emergency department (ED) evaluation or readmission. Of the 55 papers reviewed, 21 reported data on one or more of these clinical outcomes [Table 2]. Most studies graded complications using the Clavien-Dindo scale but did not report other clinical outcomes consistently. The most frequently observed complications following RMB are bleeding, hematoma, and infection. In Marconi’s meta-analysis, the median overall complication rate was 8.1% (IQR: 2.7%-11.1%), with a rate of 4.3% for perirenal hematoma (IQR: 2.7%-7.8%) and 0.70% requiring transfusion. Reported complication rates in more recent studies range from 0.5% to 13.5% (median: 4.5%)[25]. This variability highlights the need for more standardized reporting of complication outcomes to better assess and manage the risks associated with RMB.
Clinical outcomes and complications following RMB
| N | Not outpatient (observation/ admission) | ED visits | Readmissions | Overall complication rate % | Perirenal hematoma | Venous bleeding through coaxial sheet | Clinically significant pain | Gross hematuria | Pneumothorax | Urinary retention | Infection | Other | |
| Systematic reviews | |||||||||||||
| Patel et al., 2016[26] | 2,979 | NR | NR | NR | NR | 4.9% 0.4%a | 2.4% | 1.2% | 1.0% | 0.6% | NR | NR | NR |
| Marconi et al., 2016[25] | 5,228 | NR | 0 | 0.02% (1) | 8.1% (IQR: 2.7%-11.1%) | 4.3% (IQR: 2.7%-7.8%) 0.7%b | NR | 3% (IQR: 1.0%-4.8%)c | 3.15% (IQR: 1.1%-4.8%) | 0.04% (2) | 0.02% (1) | 0.02% (1) | NR |
| Retrospective studies conducted (2016-present) | |||||||||||||
| Prince et al., 2015[90] | 565 | 1.2% (7) | NR | NR | NR | 0.35% (2) | NR | NR | NR | NR | NR | NR | 0.18% (1)d 0.18% (1)e |
| Dave et al., 2017[36] | 108 | 0 | 0 | 0 | 2.8% (3) | 0.93% (1) | NR | NR | 0.93% (1) | NR | NR | NR | 0.93% (1)f |
| Alle et al., 2018[92] | 184 | 1.1% (2) | NR | NR | 7.6% (14) | NR | NR | NR | 0.5% (1) | NR | NR | NR | 0.5% (1)g 5.5% (10)h 0.5% (1)i 0.5% (1)j |
| Rasmussen et al., 2018[37] | 287 | 1.1% (3) | NR | 2 | 3.8% (11) | NR | 0.35% (1) | NR | 2% (6) | NR | NR | 0.7% (2) | 0.4% (1)k 0.4% (1)l |
| Tolouee et al., 2018[68] | 240 | 48.3% (116) | NR | 0.4% (1) | 2.1% (5) | NR | NR | NR | 0.4% (1) | 0.4% (1) | NR | 1.25% (3)m | NR |
| Garbens et al., 2021[106] | 6,840 | NR | 16.0% (1,095) | 19.1% (1,306) | 13.5% (924) | NR | NR | NR | NR | NR | NR | NR | 2.3% (159)n |
| Lobo et al., 2020[69] | 55 | 1.8% (1) | NR | NR | 1.8% (1) | NR | NR | NR | NR | NR | NR | NR | 1.8% (1)o |
| Ozambela et al., 2020[107] | 115,511 | NR | NR | NR | NR | 0.057% (66) | 0.049% (57)p | NR | 5.18% (5,983) | 1.75% (2,021) | NR | NR | 0.0034% (4)q 0.044% (51)r 0.038% (44)s |
| Santy et al., 2020[96] | 180 | NR | NR | NR | 2.2% (4) | NR | NR | NR | NR | NR | NR | NR | NR |
| Ferrari et al., 2021[99] | 84 | NR | NR | NR | 6% (5) | 6% (5) | NR | 1.2% (1)t | NR | NR | NR | 1.2% (1)u | NR |
| Patel et al., 2021[108] | 282 | NR | 2.5% (7) | 1.8% (5) | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Patel et al., 2021[39] | 72 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | NR | NR | NR |
| Srivastava et al., 2021[60] | 429 | 0 | NR | 0 | 0.5% (2) | 0.5% (2) | NR | NR | NR | NR | NR | NR | NR |
| Nazzani et al., 2022[103] | 102 | NR | NR | NR | 4.9% (5) | NR | NR | NR | NR | NR | NR | NR | NR |
| Valtersson et al., 2022[104] | 484 | 2.3% (11) | NR | 2.2% (11) | 4.5% (22) | NR | NR | NR | NR | NR | NR | NR | NR |
| Staehler et al., 2024[109] | 106 | NR | NR | NR | 5.7% (6) | NR | NR | NR | NR | NR | NR | NR | 5.7% (6)v |
| Robert et al., 2023[110] | 576 | 2.8% (16) | 0.7% (4)w | 1.7% (10)x | 4.3% (25) | 2% (12) 0.2% (1)y | 0.5% (3)z | NR | 0.3% (2) | 0.5% (3) | 0.3% (2) | 0.2% (1) | 0.3% (2)aa |
| Boynton et al., 2025[56] | 632 | NR | 3.1% (25) | 0.9% (7) | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Serhal et al., 2024[105] | 167 | NR | NR | NR | 5.4% (9) | 3% (6) | NR | 1% (1) | NR | NR | NR | 1% (1)ab | 1% (1)ac |
With respect to bleeding management, when routine imaging is performed 30-120 min after RMB, minor bleeding or developing hematomas are commonly detected and can usually be managed conservatively. Bleeding complications reported in the literature include those requiring no change in management (grade 1), and those requiring intervention, such as blood transfusion (grade 2) or a procedural intervention (grade 3). The use of hemostatic agents delivered through the coaxial sheath appears to reduce the risk of ongoing bleeding. Extended observation is warranted in cases of expanding hematoma, flank pain, or hemodynamic instability.
In nearly all studies reporting this outcome, more than 97% of RMBs were completed as outpatient procedures. Four studies reported rates of ED visits after RMB (median: 2.8%, range: 0.7%-16%), while seven reported readmission rates (median: 1.7%, range: < 0.1%-19%). These outcomes should be routinely reported in future investigations of RMB, particularly given the diverse settings in which the procedure is now performed.
Impact of RMB on initial treatment selection
Of the 55 reviewed studies, 37 reported data on the initial treatment decisions of patients who underwent RMB [Tables 3 and 4]. Thirteen of these also included treatment data for patients who did not undergo RMB. There is heterogeneity in how patients were included and how treatment selection and type were reported. Some studies described single-center experiences, while others analyzed large databases[54,55]. Most of these approaches carry substantial risks of selection bias and confounding, which must be acknowledged as they may significantly affect reported results[56].
Impact of RMB on initial treatment selection (Listed are studies in which treatment for cT1RM is mentioned for patients who underwent RMB and those who did not undergo RMB)
| N | Patients with RMB | No intervention after RMB | KSI after RMB | RN after RMB | Patients without RMB | No intervention without RMB | KSI without RMB | RN without RMB | Difference in no intervention rate (RMB vs. no RMB) | |
| Beisland et al., 2018[111] | 3,154 | 10.5% (330) | 24.3% (80) | 50.9% (168) | 24.8% (82) | 89.5% (2,824) | 3.6% (103) | 64.3% (1,815) | 32.1% (906) | +47.3% |
| Patel et al., 2020[54] | 106,258 | 11.0% (11,676) | 36.8% (39,102)a | NR | NR | 89.0% (94,582) | 11.4% (12,113)b | NR | NR | +25.4% |
| Lobo et al., 2020[69] | 83 | 66.3% (55) | 35.8% (19) | 60% (33) | 1.9% (1) | 33.7% (28) | 70.4% (19) | 28.5% (28) | 0 | -34.6% |
| Michel et al., 2020[112] | 58,225 | 8.8% (5,117) | 22.1% (1,133) | 55.7% (2,851) | 22.1% (1,133) | 91.2% (53,108) | 7.2% (3,828) | 61.2% (32,511) | 31.6% (16,769) | +14.9% |
| Shahait et al., 2020[97] | 1,597 | 11.0% (175) | 38.2% (67) | 66.6% (72) | 33.3% (36) | 89.0% (1,422) | 3.6% (51) | 64.3% (914) | 32.1% (457) | +31.6% |
| Tong et al., 2020[58] | 169 | 49.1% (83) | 20.5% (17)c | 4.8% (4)d | 74.7% (62)e | 50.9% (86) | 0 | 7.0% (6)f | 93% (80)g | +20.5% |
| Couture et al., 2021[63] | 3,838 | 25.9% (993) | 19.9% (198)h,i | 66.8% (663)h,i,j | 13.3% (132) | 74.1% (2,845) | 24.5% (696) | 62.1% (1,767)h,k,l | 13.4% (382)h,l | -4.6% |
| Patel et al., 2021[55] | 338,252 | 11.9% (40,276) | 19.9% (8,008)m | 47.8% (19,245)n | 32.3% (13,023) | 88.1% (297,976) | 6.8% (20,261)m | 36.0% (107,539)o | 57.1% (170,176) | +13.1% |
| Okhunov et al., 2021[113]; Patel et al., 2021[39] | 142 | 48.6% (69)p | 36.2% (25) | 56.5% (39)q | 7.2% (5) | 51.4% (73) | 13.7% (10) | 76.7% (56)r | 9.6% (7) | +22.5% |
| Srivastava et al., 2021[60] | 1,349 | 31.8% (429) | NR | NR | NR | 68.2% (920) | NR | 100% (920)s | NR | |
| Pasquier et al., 2022[79] | 163 | 34.3% (56) | 0 | 100% (56) | 0 | 65.6% (107) | 0 | 100% (107) | 0 | |
| Sinks et al., 2023[59] | 872t | 12.4% (108)u | 26.8% (29)v | 61.1% (66)w,x | 12.0% (13)x,y | 87.6% (764)z | 26.7% (204) | 52.7% (403)aa | 20.5% (157)ab | +0.1% |
| Boynton et al., 2025[56] | 4,062 | 19.6% (795) | 40.0% (318) | 45.2% (359)ac | 14.8% (118)2ad | 80.4% (3,267) | 48.4% (1,582) | 36.0% (1,174)ae | 16.0% (511)af | -8.4% |
| Median % (range) | 1,597 | 19.6% (8.8%-49.1%) | 26.8% (19.9%-40%) | 56.1% (4.8%-66.6%) | 22.1% (7.2%-74.7%) | 80.4% (33.7%-91.2%) | 12.6% (3.6%-21.6%) | 52.9% (7%-76.7%) | 31.6% (9.6%-93%) | +14.9% (-34.6%-+47.3%) |
Treatments selected following RMB in series, including only cT1RM with RMB
| Patients with RMB | Patients with available treatment data | No intervention after RMB | KSI after RMB | RN after RMB | |
| Neuzillet et al., 2004[114] | 88 | 86a | 27.9% (24) | 32.6% (28)b | 39.5% (34) |
| Londoño et al., 2013[87] | 126 | 126 | 50.0% (63) | 18.3% (23)b | 31.7% (40) |
| Richard et al., 2015[64] | 509 | 509 | 65.6% (334) | 23.0% (117)c,d | 11.4% (58)c,d |
| Buijs et al., 2018[115] | 95 | 95 | 0% (0) | 73.7% (70) | 26.3% (25) |
| Tolouee et al., 2018[68] | 224 | 175e | 43.4% (76)f | 36.6% (64)g | 20.0% (35) |
| Dave et al., 2017[36] | 108 | 107h | 26.2% (28) | 48.6% (52) | 25.2% (27) |
| Wang et al., 2018[65] | 106 | 106 | 59.4% (63) | 27.4% (29)c,i | 13.2% (14)c,i |
| Ma et al., 2020[66] | 85 | 70j | 30% (21) | 57.1% (40)c,k,l | 12.9% (9)c,k |
| Ozambela et al., 2020[107] | 115,511 | 115,511 | 74.3% (85,848) | 17.8% (20,503)m | 7.9% (9,160) |
| Santy et al., 2020[96] | 180 | 150n | 25.3% (38) | 57.3% (86) | 17.3% (26) |
| Bada et al., 2021[98] | 94 | 92o | 27.2% (25) | 72.8% (67)p | 0% (0) |
| Ferrari et al., 2021[99] | 84 | 61q | 31.1% (19) | 42.6% (26) | 26.2% (16) |
| Masic et al., 2021[100] | 306 | 306 | 22.5% (69) | 49.0% (150) | 28.4% (87) |
| Jiang et al., 2022[38] | 192 | 179r | 39.7% (71) | 52.5% (94)s | 7.82% (14) |
| Nazzani et al., 2022[103] | 106 | 96t | 8.3% (8) | 5.2% (5) | 86.5% (83) |
| Amaral et al., 2021[116] | 182 | 146u | 47.9% (70) | 34.9% (51) | 17.1% (25) |
| Chung et al., 2023[61] | 17 | 17v | 52.9% (9) | 35.3% (6)c,w | 11.8% (2)c |
| Branger et al., 2023[62] | 119 | 119 | 55.6% (65) | 41.9% (51)x,y | 2.6% (3)x |
| Gao et al., 2023[53] | 168 | 154z | 32.5% (50) | 53.9% (83) | 13.6% (21) |
| Staehler et al., 2024[109] | 106 | 72q | 29.2% (21) | 70.8% (51) | 0% (0) |
| Chen et al., 2024[67] | 158 | 168aa | 35.1% (59) | 55.4% (93)c,ab | 9.5% (16)c |
| Kajita et al., 2024[117] | 190 | 190 | 12.1% (23) | 87.9% (167) | 0% (0) |
| Serhal et al., 2024[105] | 167 | 149ac | 36.9% (55) | 48.3% (72) | 14.8% (22) |
| Median (range) | 32.5% (0%-74.3%) | 48.3% (5.2%-87.9%) | 13.6% (0%-86.5%) |
The inclusion criteria for patients with and without RMB were heterogeneous. Two studies[54,55] used the NCDB, a nationwide oncology outcomes database that captures over 70% of new cancer diagnoses in the United States from more than 1,500 Commission on Cancer-accredited facilities[57]. Many studies included patients older than 18 years with RCC, excluding those with clinically node-positive or metastatic disease[54-56,58]. Some had narrower criteria, such as renal masses > 4 cm[59], whereas others limited inclusion to single, clinically localized masses ≤ 4 cm (cT1a) on axial imaging[58]. Patel et al. (2021) uniquely studied 72 patients with a single tumor who underwent office-based, ultrasound-guided renal biopsy compared to 73 matched controls[39]. Another study examined the impact of a mandate for RMB; for this review, we summed pre- and post-mandate values within each treatment type[59]. One further study compared 920 patients who underwent RAPN for pT1 renal masses with 429 patients who underwent RMB for cT1 renal masses[60].
Among patients who did not receive immediate intervention, terminology varied: some studies classified these patients as having “no treatment”, others used “AS” for all, while some distinguished between AS and no treatment due to severe comorbidity or limited life expectancy[55]. For this analysis, we grouped patients who elected no treatment and those who elected AS into a single “no intervention” category. We then reported the proportions of patients in two additional cohorts: those who underwent KSI and those who underwent RN, as RN carries a greater risk of renal function loss and more substantial clinical impact compared with KSI. Reporting of intervention and nephrectomy type was inconsistent: six studies recorded only “surgery” without specifying PN or RN[59,61-67], while others provided detailed descriptions of treatment approach (percutaneous, laparoscopic, robotic, or open), nephrectomy type (PN or RN), nonsurgical therapies (cryoablation, radiofrequency or microwave ablation, radiation therapy), or other oncologic treatments[39,56,58,68].
In studies including patients both with and without RMB [Table 3], the median RMB rate was 19.6% (range: 8.8%-49.1%). The median proportion of patients with RMB whose initial treatment was no intervention was 26.8% (range: 19.9%-40%), compared with 12.6% (range: 3.6%-21.6%) for those without RMB. For patients with RMB, the median KSI rate was 56.1% (range: 4.8%-66.6%) vs. 52.9% (range: 7%-76.7%) without RMB. Median RN rates were 22.1% (range: 7.2%-74.7%) with RMB and 31.6% (9.6%-93%) without RMB. In studies limited to RMB patients [Table 4], the median rates were 32.5% (range: 0%-74.3%) for no intervention, 48.3% (range: 5.2%-87.9%) for KSI, and 13.6% (range: 0%-86.5%) for RN. Among RMB patients, the most common treatment was KSI in 22 reports (66.6%), followed by no intervention in 8 (24.2%) and RN in 3 (9.1%). Among non-RMB patients, KSI was most common (6 reports, 60%), followed by no intervention (2 reports, 20%) and RN (2 reports, 20%). Considerable variability was observed in the proportions of patients receiving no intervention, with differences between RMB and non-RMB cohorts ranging from -34.6% to +47.3%.
Of the 11 studies reporting no intervention rates with and without RMB, seven found higher rates with RMB, while three found lower rates. For example, Patel et al. (2020) reported that RMB was strongly associated with nonsurgical management in multivariable analysis (OR: 4.80, 95%CI: 4.58-5.02, P < 0.001)[54]. Similarly, the UC Irvine group found AS rates were significantly higher in RMB patients (34.7% vs. 13.7%, P < 0.001)[39]. Another NCDB analysis showed that RMB patients were more often managed with no treatment (19.9% vs. 6.8%) and TA (28.0% vs. 4.2%) compared with those without RMB (P < 0.001)[55]. Both TA (OR: 10.92; P < 0.001) and no treatment (OR: 4.81; P <0.001) were significantly associated with RMB[55]. Conversely, Boynton et al. (2024) reported lower AS rates after RMB in cT1aRM patients (42% vs. 56%), while KSI (49% vs. 37%) and RN (9.1% vs. 6.5%) were more frequent in RMB patients (P = 0.0027)[56]. Lobo et al. (2020) also found RMB increased KSI by 31.5% and decreased no treatment by 34.6%[69].
While many studies suggest RMB increases AS rates by identifying benign or indolent pathology and thereby reducing unnecessary intervention [Table 3], three reported higher AS rates in non-RMB cohorts[56,63,69]. These discrepancies likely reflect differences in patient inclusion criteria. For example, patients enter the NCDB after a cancer diagnosis, whereas MUSIC includes patients after the initial evaluation for cT1RM, regardless of diagnosis. Given the high AS rates without upfront RMB in registries such as MUSIC and DISSRM, it is plausible that RMB may not further increase AS rates in patients who have already elected AS. This contrasts with Patel et al.’s finding that RMB was significantly associated with nonsurgical management (OR: 4.80, 95%CI: 4.58-5.02, P < 0.001)[54]. Thus, the clinical impact of RMB on patients already choosing AS remains unclear.
A consistent finding across the literature is that RMB prior to surgery reduces the proportion of surgically excised benign tumors. One study reported a decrease from 23% to 3% with routine use of office-based percutaneous RMB[39]. Additionally, patients undergoing RMB were more than twice as likely to be managed with AS compared to those without RMB (35% vs. 14%, P < 0.001)[39]. Although guidelines recommend RMB prior to TA, and this rationale alone could support RMB before renal surgery, this remains a controversial issue among clinicians managing cT1RMs[22,56,70].
DISCUSSION
Impact of prior RMB on the management of cT1RM
Does RMB influence patients’ choice of AS?
Shared decision making is essential in managing patients with cT1RM. However, few studies have explored how RMB results influence decision making and patient satisfaction. In one prospective study, all patients (100%) reported that they expected RMB results to guide their treatment decisions. Among these, 64% stated they would not proceed with treatment without an RMB, while 36% were willing to do so without it[61]. Post-RMB, 86% of patients indicated that the biopsy helped guide their decision, and 71% chose a treatment based on the results[61]. Another study evaluating a mandatory institutional RMB policy prior to TA reported a marked increase in AS (from 15% to 43%) and a reduction in surgery (from 46% to 22%) following implementation in 1,202 patients[59]. Among those with positive biopsy results, 83% underwent surgery or ablation, while 17% remained on AS[59].
Study design, clinical pathways, and inclusion criteria are key to consider when interpreting these findings. Not all patients presenting with cT1RM require RMB to guide management. For example, an elderly patient with limited life expectancy may receive no intervention (which may or may not be classified as AS) for a
Evidence regarding the effect of RMB on subsequent imaging interpretation in AS is limited. Jorgenson et al. described imaging changes after ablation and SBRT but did not report examples in patients who underwent RMB alone[74]. Post-procedural hematomas, placement of hemostatic agents, and intratumoral bleeding may complicate follow-up imaging, while biopsy of complex cystic lesions may significantly alter their size and appearance.
Does RMB influence patients’ choice of thermal ablation?
All societal guidelines now recommend RMB prior to or at the time of TA[4-6]. The advantages of concomitant RMB/TA include the convenience of a single session, which may particularly benefit patients on anticoagulation therapy, those with significant comorbidities, or those facing transportation challenges. However, the disadvantages include the 20%-30% incidence of benign renal neoplasms in cT1aRM, where intervention is generally unnecessary[52]. Wells et al. reported that 20% of patients who underwent RMB and TA in the same session had benign tumors ablated, compared to only 3% in those who underwent RMB in a separate session[75]. Furthermore, evidence suggests that RMB performed solely for diagnostic purposes, rather than immediately preceding a therapeutic procedure, leads to higher diagnostic yields. Wells et al. (2017) reported that 98.6% of RMBs were diagnostic when conducted two weeks before ablation, compared with 84.3% when performed in the same session (P < 0.0001)[75]. Performing RMB in advance of ablation also helps avoid unnecessary treatment of benign tumors. Similarly, Takafuji et al. found diagnostic yields were highest when RMB was conducted before TA (91%), compared to concomitant radiofrequency (80%) or cryoablation (63%)[76]. Uhlig et al. (2021) observed that staged RMB is increasingly utilized and is associated with lower odds of admission and readmission compared with concomitant RMB/TA[77]. Finally, if adverse pathology is detected on RMB before planned TA, management can be redirected toward PN or RN.
Does RMB influence patients’ choice of surgery? Does RMB affect the difficulty of subsequent surgical treatments?
In our view, the greatest value of RMB for cT1RM lies in the potential to avoid unnecessary surgical procedures. However, we believe current evidence is insufficient to definitively support this hypothesis, given the limited quality and heterogeneity of available data [Table 3]. For example, Patel et al. analyzed 106,258 patients and found that those who underwent RMB were significantly more likely to receive nonsurgical management (36.8% vs. 11.4% in those without biopsy; OR: 4.80, 95%CI: 4.58-5.02, P < 0.001)[54]. Patel et al. (2021) also reported that routine pre-surgical RMB at their institution reduced the rate of benign neoplasms identified at PN from 22% to 3%[39]. A randomized clinical trial examining RMB in patients scheduled for surgery for cT1RM is urgently needed to address this question. Such a trial should also examine patient-centered outcomes such as treatment uncertainty, anxiety, decisional regret, and quality of life.
Some surgeons have anecdotally noted that PN after RMB may be more technically challenging due to intracapsular and/or perinephric hematomas. Doganca and Obek (2019) reported perirenal hematomas in one patient and retroperitoneal adhesions in 11 of 20 patients undergoing laparoscopic PN[78]. Among the 11 cases with “moderate” to “severe” adhesions, clinical consequences included one conversion to open PN and two cases of prolonged warm ischemia due to re-excision for initially positive margins. Pasquier et al. (2022) retrospectively examined this question and found that RMB did not increase the risk of Clavien-Dindo ≥ 2 complications, operative time, warm ischemia time, or blood loss in minimally invasive PN[79]. For RN, prior RMB generally poses little issue. For PN, typical practice involves removing all overlying perinephric fat with the tumor. Given reports of papillary RCC deposits in perinephric fat and along the biopsy tract, in such histologies, it seems prudent to also resect tissue surrounding the needle tract.
Future directions
Newer radiographic modalities may reshape the management of cT1RM
Beyond standard US, CT, and MRI for diagnosing cT1RM, several emerging imaging modalities show promise for influencing the diagnostic pathway. One such approach is multiparametric MRI (mpMRI), sometimes described as a “virtual biopsy”, as it provides tissue characterization beyond what CT can offer and without requiring a needle biopsy[15]. Algorithms using mpMRI have been developed to distinguish benign from malignant tumors and to estimate the likelihood of specific histologic subtypes[80]. The clear cell RCC likelihood score (ccLS), based on MRI-derived criteria, was developed to distinguish ccRCC from other subtypes. It classifies lesions into low-risk for ccRCC (ccLS 1-2), indeterminate (ccLS 3), and high-risk for ccRCC (ccLS 4-5)[20]. Johnson et al. (2019) reported an overall accuracy of 84% for ccLS scores 4-5 (sensitivity 79%, specificity 86%, PPV 84%, and NPV 85%) and 86% for scores 1-2 (sensitivity 68%, specificity 100%, PPV 100%, and NPV 80%)[81]. Another study demonstrated increasing PPVs with higher ccLS scores: ccLS1 (5%), ccLS2 (6%), ccLS3 (35%), ccLS4 (78%), and ccLS5 (93%)[82]. Cost analyses suggest that ccLS is more economical than RMB ($1,640 vs. $2,007 for MRI-based ccLS in patients eligible for AS, assuming no additional interpretation/reporting costs). However, costs may vary by institution[83]. Although further validation is needed before widespread implementation, ccLS has the potential to obviate RMB in some patients.
Technetium-99m sestamibi imaging may also help differentiate tumor types. Clear cell RCC and papillary RCC typically show reduced uptake compared to normal renal parenchyma, whereas oncocytomas and hybrid oncocytic-chromophobe tumors show increased uptake. An algorithm incorporating RMB, 99mTc-sestarnibi SPECT/CT, and mpMRI has been proposed[15].
Another modality, girentuximab PET-CT, targets carbonic anhydrase 9, a tumor antigen highly expressed in ccRCC[84]. In a phase 3 pre-nephrectomy trial, it achieved a sensitivity of 85.5% and a specificity of 87.0% for ccRCC. However, in “cold” lesions (seen in 27% of 284 patients with RM ≤ 3 cm), both benign and malignant non-ccRCC tumors remain part of the differential diagnosis, leaving management decisions unresolved.
Overall, advanced imaging modalities represent non-invasive alternatives to RMB that may influence initial cT1RM management. Their main limitations include cost, technological availability, need for expertise (e.g., subjective elements in ccLS scoring), and inability to reliably classify non-ccRCC subtypes (as with ccLS and girentuximab PET-CT). Ultimately, while these modalities may serve as valuable first-line tools, RMB may still be required before a definitive management decision is made.
Implications
A major concern in the treatment of cT1RM is the overtreatment of benign RM and indolent RCC. RMB is a valuable tool for distinguishing benign from malignant tumors, enabling clinicians to avoid unnecessary interventions (TA, SBRT, PN, or RN). Evidence indicates that “needless” interventions are performed without prior RMB in 20%-30% of patients with benign renal neoplasms[52,72]. This practice pattern carries both clinical and economic consequences, increasing patient morbidity and healthcare costs associated with potentially avoidable procedures[60].
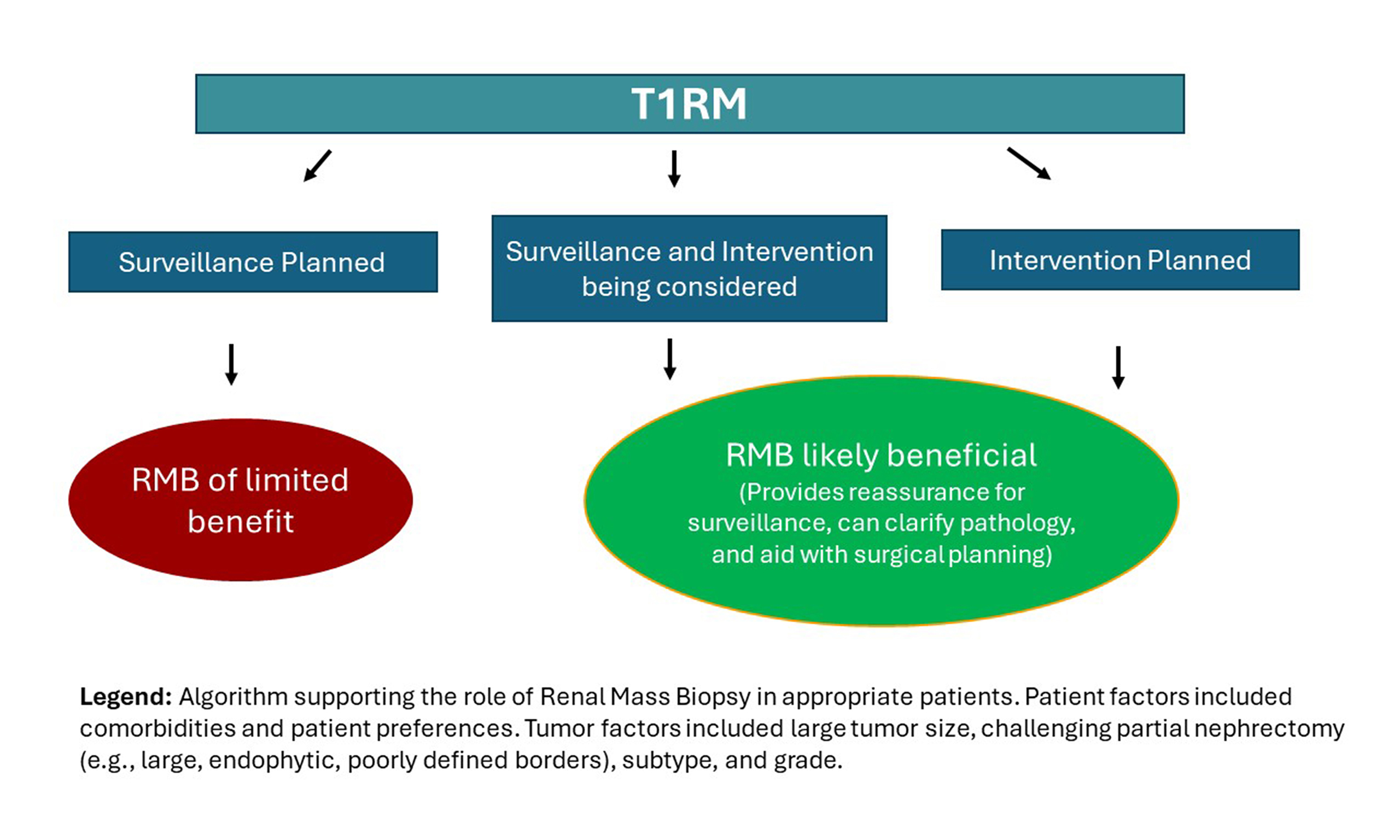
RMB also enables treatment to be tailored to the individual patient and tumor characteristics. Benign renal neoplasms can be monitored through serial imaging to confirm the absence of rapid growth. A similar approach is appropriate for indolent RCC, such as “oncocytic renal neoplasm, not further classified”, which is now recommended instead of assigning a definitive diagnosis of chromophobe RCC or oncocytoma on RMB. This also applies to other low-grade non-clear-cell RCCs, such as clear cell papillary renal neoplasm[2]. By contrast, RMB findings of ccRCC and other aggressive RCC subtypes in patients with long or intermediate life expectancy should prompt treatment, preferably with a KSI. A small proportion of patients with either complex tumor location and/or concerning pathology may require RN rather than KSI, given the latter’s potential for higher complication or recurrence rates. An RMB-first approach thus supports personalized, patient-centered management, enabling patients to make informed decisions that can positively affect their quality of life.
We hope that reports such as this, together with educational initiatives, will help dispel myths about RMB and reduce urologists’ concerns regarding its role in treatment selection, false-negative results, oncologic risks of indolent RCC, and tumor seeding rates[22,24,85]. Achieving widespread changes in RMB utilization for the diagnostic work-up of cT1RM will take much effort, with high-quality research - ideally randomized clinical trials - being a necessary next step to establish its clinical utility.
While RMB is associated with low complication rates due to the use of coaxial sheaths to decrease the number of punctures, post-procedural CT imaging, and the adjunctive use of hemostatic agents when necessary, there remains a lack of consistency in how complications are documented and reported across studies, as well as inadequate reporting of patient outcomes[86]. Establishing standardized guidelines for documenting complications of both RMB and KSIs is essential to better define RMB’s benefits and its role in clinical practice.
Overall, these findings support a paradigm shift toward evidence-based utilization of RMB to guide treatment and improve outcomes for patients with cT1RM. Developing a comprehensive framework for the appropriate timing and application of RMB will optimize its clinical benefits while minimizing risks and reducing variability in practice. Standardizing procedures and addressing existing barriers will enable the full potential of RMB to be realized, ultimately improving both patient care and resource utilization. The primary goal is to ensure that patients receive treatments offering the most favorable balance of risk, benefit, and quality of life.
In conclusion, RMB is a safe and highly effective diagnostic tool that can reduce potentially unnecessary interventions in patients with cT1RM. By providing accurate histological diagnoses, RMB supports a more individualized approach to patient care. Pathway-altering complications from RMB are minimal and rare (< 1%), underscoring its safety and supporting its broader use in patients with cT1RM compared with current practice (< 20%). Guidelines generally endorse RMB prior to TA/SBRT, but opinions differ on its necessity before initiating AS, and uncertainty remains regarding its role before PN or RN. The lack of high-level evidence regarding the utility of RMB in these settings represents a major gap in the field. Although the literature includes numerous reviews and opinion pieces on RMB, patients, care providers, and other stakeholders would benefit from more primary research - particularly studies with standardized reporting of complications and outcomes that directly compare cT1RM management with and without RMB. When updated, specialty guidelines should account for selection biases in prior studies that suggest AS is still rarely used for cT1aRM, even though prospective studies report utilization rates of about 50%. Ultimately, a randomized clinical trial that assesses the clinical impact of RMB in cT1RM management - including quality of life and other patient-centered outcomes - is the crucial next step to improving care for these patients.
DECLARATIONS
Acknowledgments
The authors gratefully acknowledge the contributions of the clinic champions, urologists, administrators, and data abstractors at each participating MUSIC practice (details around specific participating urologists and practices can be found at www.musicurology.com), as well as the members of the MUSIC Coordinating Center at the University of Michigan. Support for MUSIC is provided by Blue Cross Blue Shield of Michigan (BCBSM) through the Value Partnerships program. Although BCBSM and MUSIC collaborate, the opinions, interpretations, and conclusions expressed by the authors are their own and do not necessarily reflect those of BCBSM or its employees. The corresponding author also acknowledges the support of the Betz Family Endowment for Cancer Research (RG0813-1036). Additional funding was provided in part by the Corewell Health Foundation.
Authors’ contributions
Study conception and design, data analysis and interpretation: Solorzano MA, Omole I, Noyes SL
Conceptualization: Lane BR, Ghani KR
Methodology: Solorzano MA, Omole I, Noyes SL, Lane BR
Formal analysis: Solorzano MA, Omole I, Noyes SL, Lane BR
Data curation: Solorzano MA, Omole I, Noyes SL, Lane BR
Writing - original draft preparation: Solorzano MA, Omole I, Lane BR
Writing - review and editing: Solorzano MA, Omole I, Noyes SL, Lane BR, Rogers CG, Semerjian A
Project administration: Mirza M, Noyes SL, Lane BR
Availability of data and materials
Not applicable.
Financial support and sponsorship
Support for MUSIC is provided by Blue Cross Blue Shield of Michigan through the BCBSM Value Partnerships Program. The corresponding author also acknowledges support from the Betz Family Endowment for Cancer Research (RG0813-1036). Additional funding was provided in part by the Corewell Health Foundation.
Conflicts of interest
All authors declared that there are no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Bukavina L, Bensalah K, Bray F, et al. Epidemiology of renal cell carcinoma: 2022 update. Eur Urol. 2022;82:529-42.
2. Lim A, O’Neil B, Heilbrun ME, Dechet C, Lowrance WT. The contemporary role of renal mass biopsy in the management of small renal tumors. Front Oncol. 2012;2:106.
3. Lane BR, Babineau D, Kattan MW, et al. A preoperative prognostic nomogram for solid enhancing renal tumors 7 cm or less amenable to partial nephrectomy. J Urol. 2007;178:429-34.
4. Campbell SC, Clark PE, Chang SS, Karam JA, Souter L, Uzzo RG. Renal mass and localized renal cancer: evaluation, management, and follow-up: AUA Guideline: part I. J Urol. 2021;206:199-208.
5. Ljungberg B, Albiges L, Abu-Ghanem Y, et al. European Association of Urology Guidelines on renal cell carcinoma: the 2022 update. Eur Urol. 2022;82:399-410.
6. Motzer RJ, Jonasch E, Agarwal N, et al. NCCN Guidelines® Insights: kidney cancer, Version 2.2024. J Natl Compr Canc Netw. 2024;22:4-16.
7. Frank I, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H. Solid renal tumors: an analysis of pathological features related to tumor size. J Urol. 2003;170:2217-20.
8. Thompson RH, Kurta JM, Kaag M, et al. Tumor size is associated with malignant potential in renal cell carcinoma cases. J Urol. 2009;181:2033-6.
9. Lane BR, Golan S, Eggener S, et al. Differential use of partial nephrectomy for intermediate and high complexity tumors may explain variability in reported utilization rates. J Urol. 2013;189:2047-53.
10. Lebentrau S, Rauter S, Baumunk D, et al. Nephron sparing surgery for renal cell carcinoma up to 7 cm in the context of guideline development: a contribution of healthcare research. World J Urol. 2017;35:753-9.
11. Thompson RH, Kaag M, Vickers A, et al. Contemporary use of partial nephrectomy at a tertiary care center in the United States. J Urol. 2009;181:993-7.
12. Campbell SC, Uzzo RG, Karam JA, Chang SS, Clark PE, Souter L. Renal mass and localized renal cancer: evaluation, management, and follow-up: AUA Guideline: part II. J Urol. 2021;206:209-18.
13. Salka BR, Boynton D, Nwachukwu C, et al. Concordance of surgical treatment selection with the AUA guidelines for localized renal masses. Urol Oncol. 2025;43:394.e23-9.
14. Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part A: renal, penile, and testicular tumours. Eur Urol. 2016;70:93-105.
15. Diaz de Leon A, Davenport MS, Silverman SG, Schieda N, Cadeddu JA, Pedrosa I. Role of virtual biopsy in the management of renal masses. AJR Am J Roentgenol. 2019;212:1234-43.
16. Moch H, Amin MB, Berney DM, et al. The 2022 World Health Organization Classification of Tumours of the Urinary System and Male Genital Organs-Part A: renal, penile, and testicular tumours. Eur Urol. 2022;82:458-68.
17. Finelli A, Ismaila N, Bro B, et al. Management of small renal masses: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2017;35:668-80.
18. Wang Y, Butaney M, Wilder S, Ghani K, Rogers CG, Lane BR. The evolving management of small renal masses. Nat Rev Urol. 2024;21:406-21.
19. Hussain S, Mubeen I, Ullah N, et al. Modern diagnostic imaging technique applications and risk factors in the medical field: a review. Biomed Res Int. 2022;2022:5164970.
20. Pedrosa I, Cadeddu JA. How we do it: managing the indeterminate renal mass with the MRI clear cell likelihood score. Radiology. 2022;302:256-69.
21. Salles-Silva E, Lima EM, Amorim VB, Milito M, Parente DB. Clear cell likelihood score may improve diagnosis and management of renal masses. Abdom Radiol. 2024;49:4494-506.
22. Wolf JS. Evolving role of renal mass biopsy: myths, facts, and misconceptions. In: Leveillee RJ, Jorda M, editors. Renal mass biopsy. Cham: Springer International Publishing; 2020. pp. 1-12.
23. Ali SN, Tano Z, Landman J. The changing role of renal mass biopsy. Urol Clin North Am. 2023;50:217-25.
24. Lane BR, Samplaski MK, Herts BR, Zhou M, Novick AC, Campbell SC. Renal mass biopsy - a renaissance? J Urol. 2008;179:20-7.
25. Marconi L, Dabestani S, Lam TB, et al. Systematic review and meta-analysis of diagnostic accuracy of percutaneous renal tumour biopsy. Eur Urol. 2016;69:660-73.
26. Patel HD, Johnson MH, Pierorazio PM, et al. Diagnostic accuracy and risks of biopsy in the diagnosis of a renal mass suspicious for localized renal cell carcinoma: systematic review of the literature. J Urol. 2016;195:1340-7.
28. Leveillee RJ, Peairs S, Nields MW. Renal mass biopsy: simple techniques and optimizing navigational/targeting tools. In: Leveillee RJ, Jorda M, editors. Renal mass biopsy. Cham: Springer International Publishing; 2020. pp. 71-102.
29. Richard PO, Violette PD, Bhindi B, et al. Canadian Urological Association guideline: management of small renal masses - full-text. Can Urol Assoc J. 2022;16:E61-75.
30. Andersen MF, Norus TP. Tumor seeding with renal cell carcinoma after renal biopsy. Urol Case Rep. 2016;9:43-4.
31. Volpe A, Kachura JR, Geddie WR, et al. Techniques, safety and accuracy of sampling of renal tumors by fine needle aspiration and core biopsy. J Urol. 2007;178:379-86.
33. Mullins JK, Rodriguez R. Renal cell carcinoma seeding of a percutaneous biopsy tract. Can Urol Assoc J. 2013;7:E176-9.
34. Smith EH. Complications of percutaneous abdominal fine-needle biopsy. Review. Radiology. 1991;178:253-8.
35. Menhadji AD, Nguyen V, Okhunov Z, et al. Technique for office-based, ultrasonography-guided percutaneous biopsy of renal cortical neoplasms using a novel transducer for facilitated ultrasound targeting. BJU Int. 2016;117:948-53.
36. Dave CN, Seifman B, Chennamsetty A, et al. Office-based ultrasound-guided renal core biopsy is safe and efficacious in the management of small renal masses. Urology. 2017;102:26-30.
37. Rasmussen LR, Loft M, Nielsen TK, et al. Short-term complications for percutaneous ultrasound-guided biopsy of renal masses in adult outpatients. Acta Radiol. 2018;59:491-6.
38. Jiang P, Arada RB, Okhunov Z, et al. Multidisciplinary approach and outcomes of pretreatment small (cT1a) renal mass biopsy: single-center experience. J Endourol. 2022;36:703-11.
39. Patel RM, Okhunov Z, Jiang P, Tapiero S, Landman J. Office-based renal tumor biopsy: a paradigm change in the management of a small renal mass? Curr Urol Rep. 2021;22:43.
40. Faraj K, Dave CN, Patel K, et al. A retrospective comparative outcomes and cost analysis of office based, ultrasound guided renal mass biopsy performed by urologists and standard hospital biopsies for small renal masses. Urol Pract. 2018;5:260-5.
41. Patel RM, Safiullah S, Okhunov Z, et al. Pretreatment diagnosis of the small renal mass: status of renal biopsy in the United States of America. J Endourol. 2018;32:884-90.
42. Khan AA, Shergill IS, Quereshi S, Arya M, Vandal MT, Gujral SS. Percutaneous needle biopsy for indeterminate renal masses: a national survey of UK consultant urologists. BMC Urol. 2007;7:10.
43. Jiang P, Ali SN, Peta A, et al. A review of the recommendations and strength of evidence for clinical practice guidelines on the management of small renal masses. J Endourol. 2023;37:903-13.
44. Patel HD, Druskin SC, Rowe SP, Pierorazio PM, Gorin MA, Allaf ME. Surgical histopathology for suspected oncocytoma on renal mass biopsy: a systematic review and meta-analysis. BJU Int. 2017;119:661-6.
45. Abel EJ, Culp SH, Matin SF, et al. Percutaneous biopsy of primary tumor in metastatic renal cell carcinoma to predict high risk pathological features: comparison with nephrectomy assessment. J Urol. 2010;184:1877-81.
46. Al Nazer M, Mourad WA. Successful grading of renal-cell carcinoma in fine-needle aspirates. Diagn Cytopathol. 2000;22:223-6. Available from: https://doi.org/10.1002/(SICI)1097-0339(200004)22:4%3C223::AID-DC4%3E3.0.CO;2-B. [Last accessed on 17 Sep 2025].
47. Ball MW, Bezerra SM, Gorin MA, et al. Grade heterogeneity in small renal masses: potential implications for renal mass biopsy. J Urol. 2015;193:36-40.
48. Nahouraii LM, Allen JL, Merrill SB, Lehman E, Kaag MG, Raman JD. Histologic heterogeneity of extirpated renal cell carcinoma specimens: implications for renal mass biopsy. J Kidney Cancer VHL. 2020;7:20-5.
49. Millet I, Curros F, Serre I, Taourel P, Thuret R. Can renal biopsy accurately predict histological subtype and Fuhrman grade of renal cell carcinoma? J Urol. 2012;188:1690-4.
50. Blumenfeld AJ, Guru K, Fuchs GJ, Kim HL. Percutaneous biopsy of renal cell carcinoma underestimates nuclear grade. Urology. 2010;76:610-3.
51. Johnson DC, Vukina J, Smith AB, et al. Preoperatively misclassified, surgically removed benign renal masses: a systematic review of surgical series and United States population level burden estimate. J Urol. 2015;193:30-5.
52. Kim JH, Li S, Khandwala Y, Chung KJ, Park HK, Chung BI. Association of prevalence of benign pathologic findings after partial nephrectomy with preoperative imaging patterns in the United States from 2007 to 2014. JAMA Surg. 2019;154:225-31.
53. Gao H, Nowroozizadeh B, Zepeda JP, et al. The success rate of small renal mass core needle biopsy and its impact on lowering benign resection rate. BMC Urol. 2023;23:189.
54. Patel HD, Nichols PE, Su ZT, et al. Renal mass biopsy is associated with reduction in surgery for early-stage kidney cancer. Urology. 2020;135:76-81.
55. Patel DN, Ghali F, Meagher MF, et al. Utilization of renal mass biopsy in patients with localized renal cell carcinoma: a population-based study utilizing the National Cancer Database. Urol Oncol. 2021;39:79.e1-8.
56. Boynton DN, Mirza M, Van Til M, et al. Renal mass biopsy is associated with fewer radical nephrectomies for benign or indolent disease, particularly for T1b renal masses. Urol Pract. 2025;12:148-56.
57. Volpe A, Finelli A, Gill IS, et al. Rationale for percutaneous biopsy and histologic characterisation of renal tumours. Eur Urol. 2012;62:491-504.
58. Tong W, Lin X, Xu Y, Yan Y. The role of percutaneous fine needle aspiration biopsy in the management of small renal masses without chance of nephron-sparing surgery. Int Urol Nephrol. 2020;52:2223-8.
59. Sinks A, Miller C, Holck H, et al. Renal mass biopsy mandate is associated with change in treatment decisions. J Urol. 2023;210:72-8.
60. Srivastava A, Uzzo RN, Lee J, et al. Renal mass biopsy: a strategy to reduce associated costs and morbidity when managing localized renal masses. Urol Oncol. 2021;39:790.e9-15.
61. Chung R, Kurtzman JT, Gillespie A, et al. The utility of renal mass biopsy in shared decision-making for renal mass treatment. Urology. 2023;178:98-104.
62. Branger N, Bigot P, Pignot G, et al. Oncocytoma on renal mass biopsy: is it still the same histology when surgery is performed? Results from UroCCR-104 study. World J Urol. 2023;41:483-9.
63. Couture F, Finelli T, Breau RH, et al. The increasing use of renal tumor biopsy amongst Canadian urologists: when is biopsy most utilized? Urol Oncol. 2021;39:499.e15-22.
64. Richard PO, Jewett MA, Bhatt JR, et al. Renal tumor biopsy for small renal masses: a single-center 13-year experience. Eur Urol. 2015;68:1007-13.
65. Wang X, Lv Y, Xu Z, et al. Accuracy and safety of ultrasound-guided percutaneous needle core biopsy of renal masses: a single center experience in China. Medicine. 2018;97:e0178.
66. Ma LX, Craig KM, Mosquera JM, et al. Contemporary results and clinical utility of renal mass biopsies in the setting of ablative therapy: a single center experience. Cancer Treat Res Commun. 2020;25:100209.
67. Chen S, Chen Y, Li J, et al. Accuracy, safety, and diagnostic prediction of percutaneous renal mass biopsy and subsequent changes in treatment. Wideochir Inne Tech Maloinwazyjne. 2024;19:113-21.
68. Tolouee SA, Madsen M, Berg KD, Dahl C, Fode M, Azawi NH. Renal tumor biopsies are associated with a low complication rate. Scand J Urol. 2018;52:407-10.
69. Lobo JM, Clements MB, Bitner DP, et al. Does renal mass biopsy influence multidisciplinary treatment recommendations? Scand J Urol. 2020;54:27-32.
70. Kutikov A, Smaldone MC, Uzzo RG, Haifler M, Bratslavsky G, Leibovich BC. Renal mass biopsy: always, sometimes, or never? Eur Urol. 2016;70:403-6.
71. Menon AR, Hussein AA, Attwood KM, et al. Active surveillance for risk stratification of all small renal masses lacking predefined clinical criteria for intervention. J Urol. 2021;206:229-39.
72. Gao B, Gorgen ARH, Bhatt R, et al. Avoiding “Needless” nephrectomy: what is the role of small renal mass biopsy in 2024? Urol Oncol. 2024;42:236-44.
73. Ambani SN, Morgan TM, Montgomery JS, et al. Predictors of delayed intervention for patients on active surveillance for small renal masses: does renal mass biopsy influence our decision? Urology. 2016;98:88-96.
74. Jorgenson LC, Navin PJ, Adamo D, et al. Imaging and nonsurgical management of renal masses. Radiographics. 2025;45:e240093.
75. Wells SA, Wong VK, Wittmann TA, et al. Renal mass biopsy and thermal ablation: should biopsy be performed before or during the ablation procedure? Abdom Radiol. 2017;42:1773-80.
76. Takafuji M, Fujimori M, Nakatsuka A, et al. Computed tomography-guided biopsy for small renal masses before or immediately after tumor ablation: factors affecting diagnostic yield. Jpn J Radiol. 2021;39:283-92.
77. Uhlig A, Lenis A, Wang X, Shuch B. Sequencing of renal mass biopsy and ablation: results from the National Cancer Database. Urol Pract. 2021;8:555-64.
78. Doganca T, Obek C. Evaluation of diagnostic accuracy of percutaneous biopsy for small renal masses and first report of post-biopsy adhesions: a prospective study. Urol J. 2019;16:357-60.
79. Pasquier D, Rozet F, Fregeville A, et al. Renal tumor biopsy does not increase the risk of surgical complications of minimally invasive partial nephrectomy. Prog Urol. 2022;32:843-8.
80. Kumar S, Virarkar M, Vulasala SSR, et al. Magnetic resonance imaging virtual biopsy of common solid renal masses - a pictorial review. J Comput Assist Tomogr. 2023;47:186-98.
81. Johnson BA, Kim S, Steinberg RL, de Leon AD, Pedrosa I, Cadeddu JA. Diagnostic performance of prospectively assigned clear cell Likelihood scores (ccLS) in small renal masses at multiparametric magnetic resonance imaging. Urol Oncol. 2019;37:941-6.
82. Steinberg RL, Rasmussen RG, Johnson BA, et al. Prospective performance of clear cell likelihood scores (ccLS) in renal masses evaluated with multiparametric magnetic resonance imaging. Eur Radiol. 2021;31:314-24.
83. Chen KY, Lange MJ, Qiu JX, et al. Cost-effectiveness analysis of the clear cell likelihood score against renal mass biopsy for evaluating small renal masses. Urology. 2024;188:111-7.
84. Shuch B, Pantuck AJ, Bernhard JC, et al. [89Zr]Zr-girentuximab for PET-CT imaging of clear-cell renal cell carcinoma: a prospective, open-label, multicentre, phase 3 trial. Lancet Oncol. 2024;25:1277-87.
85. Barwari K, de la Rosette JJ, Laguna MP. The penetration of renal mass biopsy in daily practice: a survey among urologists. J Endourol. 2012;26:737-47.
86. Curci N, Caoili EM. The current role of biopsy in the diagnosis of renal tumors. Semin Ultrasound CT MR. 2017;38:72-7.
87. Londoño DC, Wuerstle MC, Thomas AA, et al. Accuracy and implications of percutaneous renal biopsy in the management of renal masses. Perm J. 2013;17:4-7.
88. Bernhard JC, Bigot P, Pignot G, et al; NEPHRON Study Group. The accuracy of renal tumor biopsy: analysis from a national prospective study. World J Urol. 2015;33:1205-11.
89. Garnon J, Schlier A, Buy X, et al. Evaluation of percutaneous biopsies of renal masses under MRI-guidance: a retrospective study about 26 cases. Eur Radiol. 2015;25:617-23.
90. Prince J, Bultman E, Hinshaw L, et al. Patient and tumor characteristics can predict nondiagnostic renal mass biopsy findings. J Urol. 2015;193:1899-904.
91. Jeon HG, Seo SI, Jeong BC, et al. Percutaneous kidney biopsy for a small renal mass: a critical appraisal of results. J Urol. 2016;195:568-73.
92. Alle N, Tan N, Huss J, Huang J, Pantuck A, Raman SS. Percutaneous image-guided core biopsy of solid renal masses: analysis of safety, efficacy, pathologic interpretation, and clinical significance. Abdom Radiol. 2018;43:1813-9.
93. Tsang Mui Chung MS, Maxwell AW, Wang LJ, Mayo-Smith WW, Dupuy DE. Should renal mass biopsy be performed prior to or concomitantly with thermal ablation? J Vasc Interv Radiol. 2018;29:1240-4.
94. Posielski NM, Bui A, Wells SA, et al. Risk factors for complications and nondiagnostic results following 1,155 consecutive percutaneous core renal mass biopsies. J Urol. 2019;201:1080-7.
95. Gillis CJ, Rendon R, MacDonald LP, et al. Identification of tumor size as the only factor associated with nondiagnostic biopsies in patients with small renal masses. Can Urol Assoc J. 2020;14:E220-3.
96. Santy A, Basset V, Colleter L, et al. Operative and oncological results and impact on treatment strategy of systematic renal tumor biopsy: comparison between patients < and >75 years old. Urol Oncol. 2020;38:74.e21-7.
97. Shahait M, Jackman S, Landman J, et al. Utilization and operative influence of renal mass biopsy in the small renal mass: analysis from the Clinical Research Office of the Endourological Society Small Renal Mass Registry. J Endourol. 2020;34:99-106.
98. Bada M, Rapisarda S, Cicero C, et al. The role of renal biopsy to improve small renal mass diagnosis and management: are there predictive factors for a higher detection rate?. The first Italian study of 100 cases. Minerva Urol Nephrol. 2021;73:78-83.
99. Ferrari M, Cartolari R, Barizzi J, Pereira Mestre R, D’Antonio E, Renard J. Percutaneous biopsy of small renal mass: can diagnostic accuracy be affected by hospital volume? Cent European J Urol. 2021;74:334-40.
100. Masic S, Strother M, Kidd LC, et al. Feasibility and outcomes of renal mass biopsy for anatomically complex renal tumors. Urology. 2021;158:125-30.
101. Jasinski M, Siekiera J, Tworkiewicz M. Ultrasound-guided renal mass biopsy and its clinical utility: a single-centre experience. Urol Int. 2022;106:560-6.
102. Kapur P, Setoodeh S, Araj E, et al. Improving renal tumor biopsy prognostication with BAP1 analyses. Arch Pathol Lab Med. 2022;146:154-65.
103. Nazzani S, Zaborra C, Biasoni D, et al. Renal tumor biopsy in patients with cT1b-T4-M0 disease susceptible to radical nephrectomy: analysis of safety, accuracy and clinical impact on definitive management. Scand J Urol. 2022;56:367-72.
104. Valtersson J, Rasmussen BS, Elgborn A, Lund L, Graumann O. One hour observation of patients after image-guided percutaneous renal mass biopsy. Acta Radiol Open. 2022;11:20584601221138555.
105. Serhal M, Rangwani S, Seedial SM, et al. Safety and diagnostic efficacy of image-guided biopsy of small renal masses. Cancers. 2024;16:835.
106. Garbens A, Wallis CJD, Klaassen Z, et al. Comprehensive assessment of the morbidity of renal mass biopsy: a population-based assessment of biopsy-related complications. Can Urol Assoc J. 2021;15:42-7.
107. Ozambela M Jr. , Wang Y, Leow JJ, Silverman SG, Chung BI, Chang SL. Contemporary trends in percutaneous renal mass biopsy utilization in the United States. Urol Oncol. 2020;38:835-43.
108. Patel AK, Lane BR, Chintalapati P, et al. Utilization of renal mass biopsy for T1 renal lesions across michigan: results from MUSIC-KIDNEY, a statewide quality improvement collaborative. Eur Urol Open Sci. 2021;30:37-43.
109. Staehler M, Rodler S, Brinkmann I, et al. Long-term follow-up in patients undergoing renal mass biopsy: seeding is not anecdotal. Clin Genitourin Cancer. 2024;22:189-92.
110. Robert SC, Cossetto T, Miao TL, et al. Complications after renal mass biopsy: frequency, nature, timing, and associated characteristics. AJR Am J Roentgenol. 2023;221:344-53.
111. Beisland C, Johannesen TB, Reisæter LAR, Hjelle KM. Real-life use of diagnostic biopsies before treatment of kidney cancer: results from a Norwegian population-based study. Scand J Urol. 2018;52:38-44.
112. Michel J, Lenis AT, Lec PM, et al. Analysis of guideline recommended use of renal mass biopsy and association with treatment. Can J Urol. 2020;27:10285-93.
113. Okhunov Z, Gorin MA, Jefferson FA, et al. Can preoperative renal mass biopsy change clinical practice and reduce surgical intervention for small renal masses? Urol Oncol. 2021;39:735.e17-23.
114. Neuzillet Y, Lechevallier E, Andre M, Daniel L, Coulange C. Accuracy and clinical role of fine needle percutaneous biopsy with computerized tomography guidance of small (less than 4.0 cm) renal masses. J Urol. 2004;171:1802-5.
115. Buijs M, Wagstaff PGK, de Bruin DM, et al. An in-vivo prospective study of the diagnostic yield and accuracy of optical biopsy compared with conventional renal mass biopsy for the diagnosis of renal cell carcinoma: the interim analysis. Eur Urol Focus. 2018;4:978-85.
116. Amaral BS, Macek P, Arora A, et al. Renal tumor biopsy: rationale to avoid surgery in small renal masses. Curr Urol Rep. 2021;22:46.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Special Issue
Copyright
Data & Comments
Data
















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.