Cancer-associated O-glycans and microbiome interactions in colorectal cancer: insights into tumor progression and immune evasion
Abstract
Glycans play a crucial role in modulating cellular interactions and disease progression. In the colon, they serve as key mediators between host cells, the microbiome, and the immune system. During tumorigenesis, however, glycans undergo significant alterations that not only influence oncogenic pathways but are also affected by changes in cell signaling, creating a self-perpetuating cycle. These feedback loops drive several cancer hallmarks, including sustained proliferative signaling and immune escape, thereby promoting disease progression. One prominent alteration in colorectal cancer is increased sialylation - the enrichment of sialic acid-containing glycans - which is strongly linked to tumor development, progression, and poor prognosis. Truncated O-glycan structures, such as the Sialyl-Tn (STn) antigen, are rarely presented in healthy colon tissue but are commonly associated with oncogenic transformation and immune evasion. Both commensal and pathogenic bacteria in the colon exploit host sialylated glycans as adhesion sites and nutrient sources. This interaction modulates local immune responses and inflammation, contributing to a complex and dynamic interplay that, when disrupted, accelerates cancer progression. This mini-review discusses the role of sialylated cancer-associated glycans in colorectal cancer, emphasising their involvement in tumor progression, metastasis, and interactions with the gut microbiome. Furthermore, it highlights emerging therapeutic strategies that target these glycans.
Keywords
INTRODUCTION
The glycocalyx, a carbohydrate-rich layer present on all cell types, plays a crucial role in cellular communication, recognition, and intracellular signaling[1]. Composed of diverse glycans - either free or conjugated to proteins and lipids - this dynamic structure is essential for maintaining cellular functions and responding to environmental cues. The glycome, which represents the complete set of glycans within a cell or tissue, acts as a molecular fingerprint that reflects tissue identity and adapts to both physiological and pathological changes[2].
Protein glycosylation varies among cell types and microenvironments due to differences in carbohydrate availability and the activity of glycosylation enzymes[3]. Unlike DNA-encoded processes, glycosylation is not template-driven. Instead, the glycan structures present on a given cell depend on the expression of multiple genes encoding glycan-modifying enzymes such as glycosyltransferases and glycosidases[4]. Enzyme expression, specificity, location, and substrate variability collectively shape the diversity of the cellular glycome.
In humans, protein glycosylation starts during translation in the endoplasmic reticulum and continues in the Golgi apparatus, resulting in N- and O-linked glycosylation[5]. Other forms include proteoglycans with glycosaminoglycans, glycosylphosphatidylinositol-anchored proteins, and O-GlcNAc modifications[6]. The structural complexity and diverse biological roles of glycans highlight their importance as potential prognostic markers and therapeutic targets.
Dysregulation of glycans has been linked to various pathological conditions, particularly cancer. Cancer-associated glycans contribute to several hallmarks of the disease, enhancing cancer cell adaptation and survival[7]. Glycosylation is highly dynamic during tumor progression, and abnormal glycan structures typically promote tumor growth and metastasis by bypassing regulatory checkpoints, preventing apoptosis, and facilitating immune evasion[8]. Consequently, glycans hold promise as both biomarkers for specific cancers and as potential therapeutic targets[8].
Beyond cancer, glycans also play a central role in host-microbe interactions. Host cells deploy glycans, such as those on mucins, to trap pathogens, aid immune cells in their elimination, or serve as adhesion points that facilitate infection. Host glycans can additionally provide a nutrient source for commensal bacteria, which possess specialized binding and degradation machinery that allows them to thrive in mucin-rich environments. The interplay between host glycans and the microbiome is highly complex, with the microbiome also modulating host-glycan expression and metabolism[9]. Both commensal and pathogenic microbes can modify host glycans, thereby influencing immune responses, homeostasis, and disease progression. Dysbiosis - an imbalance in the microbial community - can lead to aberrant glycosylation patterns, disrupting host-microbe interactions and contributing to chronic inflammation and oncogenesis[10]. Moreover, cancer-associated changes in glycosylation can reshape microbial composition and metabolic activity in ways that support tumor growth and immune evasion[11-13].
SIALYLATION AS A CRITICAL MODULATOR OF PHYSIOLOGICAL FUNCTIONS AND DISEASE PATHOGENESIS
Sialylation, the addition of sialic acid to the terminal position of a glycan chain, is a common modification of glycoproteins. Sialic acids are a family of monosaccharides located at the termini of glycans on proteins and lipids, with N-acetylneuraminic acid (Neu5Ac) being the predominant form in humans. These residues are typically linked to other monosaccharides via α2,3-, α2,6-, or α2,8-linkages. In humans, sialylation profiles are regulated by twenty sialyltransferases, which add sialic acids and four neuraminidases, which remove them. Both enzyme families exhibit tissue-specific expression and substrate preferences.
Sialylation plays a crucial role in regulating both physiological processes and disease mechanisms. One key function is its role as a checkpoint inhibitor of innate immune responses, mediated through recognition by immune inhibitory receptors known as sialic acid-binding immunoglobulin-like lectins (Siglecs). Humans express 14 Siglecs, classified into two main groups based upon phylogenetic relationships. The first group includes Siglec-1 (sialoadhesion), Siglec-2 (CD22), Siglec-4 (MAG), and Siglec-15[14-16]. The second group comprises the CD33-related Siglecs, including Siglec-3 (CD33), -5, -6, -7, -8, -9, -10, -11, -14, and -16[17]. The extracellular domains of Siglecs contain a V-set N-terminal Ig-like domain, which includes a carbohydrate recognition domain (CRD) that binds sialylated glycans and confers distinct binding specificities[18]. Most Siglecs possess cytoplasmic immunoreceptor tyrosine-based inhibition motifs (ITIMs) that can be phosphorylated by Src family kinases[19], enabling high-affinity interaction with SHP-1 and SHP-2 phosphatases, which in turn inhibit downstream signaling pathways[20]. Through these interactions, Siglecs promote immunosuppressive signaling and help tumor cells evade immune surveillance[21]. For example, the recognition of sialylated glycans on cancer cells by Siglec-7/Siglec-9 expressed on natural killer (NK) cells suppresses NK-mediated cytotoxicity[22]. Similarly, the engagement of Siglec-9 on macrophages with its sialylated ligands on breast cancer cells induces a tumor-associated macrophage phenotype, promoting the release of factors that drive cancer progression[23]. Glioma cells have also been shown to express sialylated ligands for several Siglecs (Siglec-3, -5, -7, and -9), thereby facilitating evasion from myeloid-derived suppressor cells[23].
Sialic acids also modulate cell signaling by influencing the function of membrane receptors decorated with sialylated glycans. For example, we reported that sialic acids regulate dendritic cell activity, including antigen presentation and T cell interactions, by modulating MHC-I turnover[24,25]. Another example is found in immunoglobulins, where sialylation of the Fc region enhances anti-inflammatory properties by altering receptor recognition[26]. Moreover, sialylation affects the plasma levels and half-life of immunoglobulins and other proteins. Taken together, these findings highlight that sialylation exerts broad and profound effects on cellular and molecular functions, making it a critical determinant of both physiological processes and disease pathogenesis.
Impact of aberrant sialylation in colorectal cancer
Altered sialylation is a hallmark of cancer, contributing to metastasis, immune evasion, and dysregulated signaling[7]. These changes often result from elevated expression of sialyltransferases, downregulation of neuraminidases, the combined effects of both, or their abnormal subcellular localization[27]. The twenty sialyltransferases expressed in humans reside in the medial or trans cisternae of the Golgi apparatus. They are divided into four classes based on substrate specificity and linkage type: ST3Gal (ST3 Beta-Galactoside Alpha-2,3-Sialyltransferase), ST6Gal (ST6 Beta-Galactoside Alpha-2,6-Sialyltransferase), ST6GalNAc (ST6 N-acetylgalactosaminide alpha-2,6-sialyltransferase), and ST8Sia (ST8 alpha-N-acetyl-neuraminide alpha-2,8-sialyltransferase)[28]. The overexpression of ST3Gal1, ST3Gal2, and ST6GalNAc2 was first reported in colorectal cancer (CRC) and linked to poor prognosis[29]. In addition, impaired neuraminidase activity further increases α2,6-sialylation in colon tumors, reinforcing a hypersialylated phenotype[29].
Aberrant sialylation plays a multifaceted role in CRC tumorigenesis. It reduces patient survival by promoting metastasis and facilitates immune escape by engaging inhibitory Siglec receptors on immune cells. Sialylated glycans are recognised as “self-antigens” through Siglec binding, triggering inhibitory signaling cascades that suppress immune activation. This form of “molecular mimicry” allows tumor cells to proliferate and metastasize undetected by the immune system.
A distinctive feature of CRC is the expression of truncated, sialylated O-glycans - most notably the Sialyl-Thomsen-nouveau (STn) antigen. While normal colon tissue exhibits complex, highly branched O-glycan structures, STn is a disaccharide consisting of sialic acid linked to N-acetylgalactosamine (GalNAc), detected in 93%-96% of CRC but not in healthy tissues[30,31]. STn promotes proliferative signaling by modifying tumor-associated receptors such as Muc1 and CD44. It also induces tolerogenic immune responses through recognition by inhibitory receptors on dendritic cells[32] and via crosstalk with M2 protumor macrophages[30]. STn expression has been attributed to elevated levels or mislocalization of ST6GalNAc1, the sialyltransferase responsible for transferring sialic acid to N-acetylgalactosamine[31]. COSMC (Core 1 β3-Gal-T-Specific Molecular Chaperone, C1GalT1C1) also plays a critical role in regulating STn expression. Dysfunctional COSMC causes aggregation and proteasomal degradation of T-synthase, resulting in Tn-antigen accumulation and aberrant O-glycosylation, a feature observed in various malignancies including CRC[33]. Gene mutations in COSMC - such as deletions or promoter hypermethylation - are major mechanisms underlying aberrant O-glycosylation in tumors[34]. Moreover, STn expression is normally masked by O-acetylation and undetectable in healthy tissues, but becomes elevated during malignant progression, where it correlates with lymphatic and venous invasion[35,36].
Beyond STn, sialyl Lewis antigens are frequently reported in CRC. Advanced cancers and metastatic lesions show 94%-96% overexpression of sialyl Lewis X (sLex) and sialyl Lewis A (sLea), along with increased α2-3 and α2-6 sialylation, all of which correlate with metastatic potential[37,38]. sLexand sLea are tetrasaccharide isomers composed of sialic acid, galactose, fucose, and N-acetylglucosamine. Elevated serum levels of these antigens are clinically exploited, with sLea (CA19-9) serving as a well-known cancer biomarker. Both STn and sLex/a antigens contribute to tumor progression, immune escape, and metastasis by altering cell-cell interactions and signaling pathways[39].
In addition to these well-characterized sialylated antigens, several other glycosylation modifications, including α2,3 and α2,6-sialylation, have been reported across gastrointestinal cancers. These alterations, along with their biosynthetic enzymes, are summarized in Table 1.
Glycosylation alterations, associated enzymes and effects in colorectal cancer
| Glycan pattern | Responsible enzyme | Associated effects | References |
| Core-3 O-glycan alterations | Core-3 synthase | Promotes chronic inflammation; facilitates tumor progression and metastasis | [40,41] |
| α2,6-Sialylation | ST6Gal1 | Overexpression linked to poor prognosis, increased invasion, and metastasis | [42] |
| Sialyl-Lewis X Antigen | FUT6, ST3Gal4 | Increased expression associated with advanced tumor stages, low survival, and selectin-mediated metastasis | [43,44] |
| Sialyl-Lewis A Antigen | FUT3, ST3Gal4 | Overexpression promotes lympho-hematogenous metastasis and poor prognosis | [45,46] |
| T-antigen (core 1) | C1GALT1 | Drives epithelial-mesenchymal transition, tumor progression, and metastasis | [47] |
| Sialyl-Tn antigen | ST6GalNAc1 | Enhances tumor progression and immune evasion through Siglec receptor interactions | [48,49] |
Sialylation and host-gut microbiome interplay
Glycosylation, including sialylation, plays a pivotal role in host-microbiome interactions within the gastrointestinal (GI) tract, influencing both physiological homeostasis and disease progression[50]. Sialylation governs key aspects of epithelial barrier function, immune regulation, and microbial dynamics. The metabolism of sialic acid is tightly regulated by both host glycosyltransferases and microbial enzymes, creating a dynamic interface that shapes the mucosal environment.
Gut microorganisms actively remodel the host glycan landscape through sialidases, glycosyltransferases, and glycan-binding proteins, thereby altering the availability and presentation of sialylated structures[51]. This microbial activity establishes a feedback loop that fine-tunes colonization, immune evasion, and nutrient acquisition, ultimately supporting gut homeostasis[52]. Both commensal and pathogenic bacteria can sense and respond to the sialylation status of host mucins, underscoring the adaptive co-evolution of host glycobiology and the gut microbiome.
Within the intestinal mucosa, sialylated mucins such as MUC2 are central to maintaining epithelial barrier integrity [Figure 1]. The terminal sialic acid residues confer strong negative charges and high hydrophilicity, facilitating the formation of a dense, hydrated mucus layer. This gel-like barrier limits microbial penetration, spatially segregates commensals, and shields epithelial surfaces from inflammatory damage[53]. O-sialylated glycans also contribute to the viscoelastic properties of mucus, resisting bacterial degradation and maintaining a stable niche for beneficial microbes[50-54].
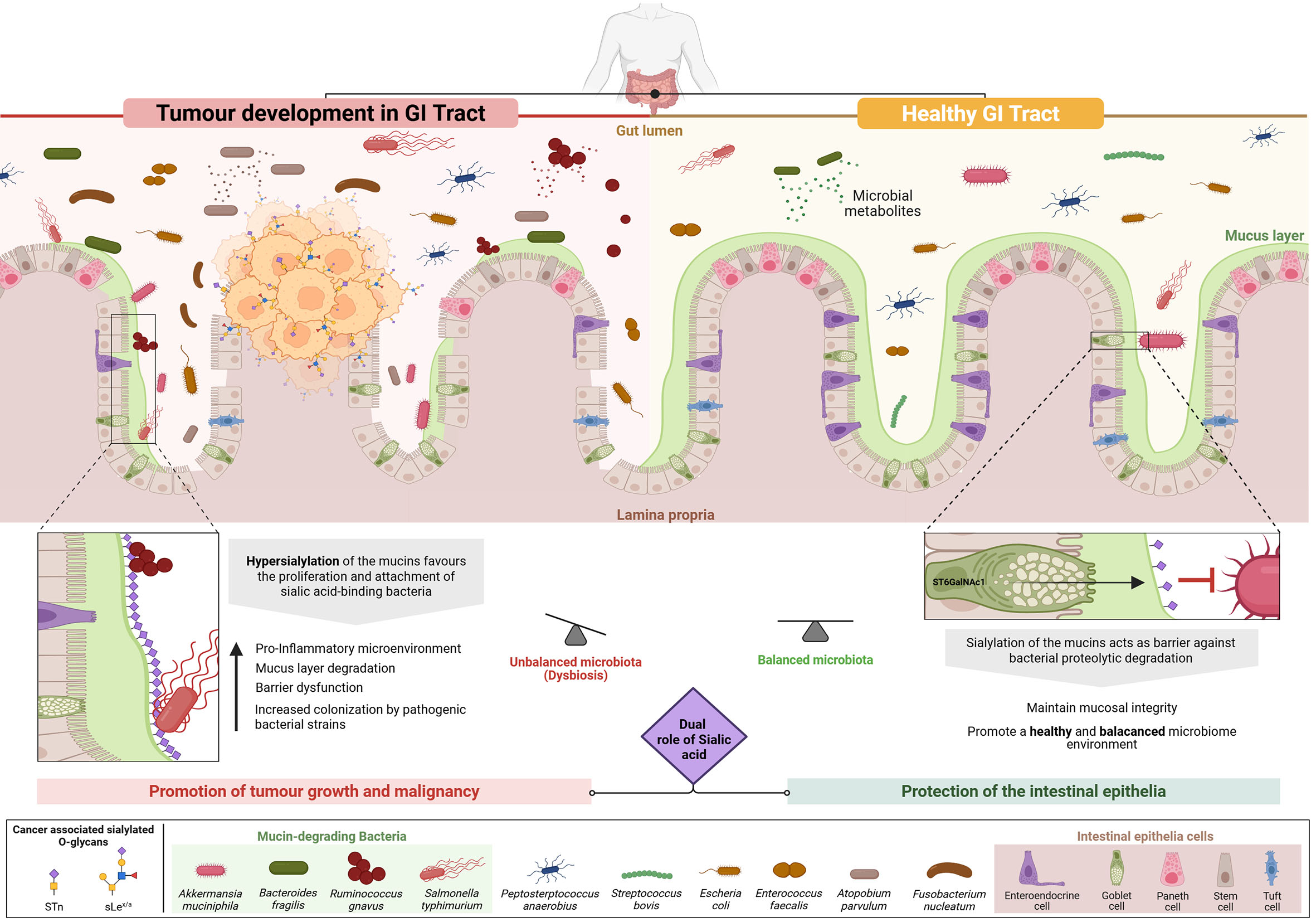
Figure 1. The Interplay of sialylated glycans with the gastrointestinal (GI) microbiota and their dual role in physiological and pathological conditions. Mucins are highly O-glycosylated proteins and represent the main functional component of the mucosal layer - the body’s first line of defense in the GI tract. Sialylation of the GI mucosa by intestinal epithelial cells is critical for maintaining homeostasis, contributing to mucus hydration, lubrication, microbial barrier functions, and immune modulation. In a healthy GI tract (right side of figure), sialylated glycans on mucins protect the intestinal epithelium by preventing degradation by mucin-degrading bacteria and by supporting balanced microbiota colonization. In contrast, under pathological conditions such as CRC (left side of figure), sialic acids can promote tumor development and progression. Tumor cells frequently express aberrant sialylated O-glycans (e.g., sialyl-Tn, sialyl Lewis x/a), which enable immune evasion (immunotolerance) and foster a chronically inflamed tumor microenvironment. This inflammatory state not only supports tumor growth, but also induces microbiota dysbiosis by favoring the proliferation and colonization of pathogenic bacteria, particularly sialic acid-binding species that attach to tumor-associated hypersialylated mucins. In turn, these microbes exacerbate inflammation and compromise the mucin barrier by degrading this protective layer, thereby facilitating tumor expansion and metastatic behavior in the GI tract. Overall, this figure illustrates the complex, context-dependent duality of sialylation - protective under physiological conditions, but pro-tumorigenic in CRC. Image created using BioRender (www.biorender.com).
While sialylated mucins sustain microbial balance and mucosal integrity, some pathogens exploit sialic acid-binding proteins to anchor to host surfaces and promote infection[54]. For example, H. pylori adheres to fucosylated and sialylated glycans in the upper digestive tract, triggering chronic inflammation and tumorigenesis[55].
Disruption of mucin sialylation, whether through desialylation or hypersialylation, has profound implications for mucosal function and microbial ecology. Desialylation caused by mutations in sialyltransferase genes such as ST6GalNAc1 and B3GALT5 compromises mucus barrier structure. This increases epithelial permeability, microbial translocation, and immune activation, contributing to chronic inflammation in Inflammatory Bowel Disease (IBD)[56,57]. In ST6GalNAc1-/- and B3Galt5-/- mouse models, loss of mucin sialylation correlates with an expansion of Proteobacteria such as Sutterella and Desulfovibrio, taxa associated with gut inflammation in both murine and human contexts[50]. Conversely, hypersialylation - characterized by excessive addition of sialic acid to mucin O-glycans - is frequently observed in CRC. Upregulation of sialyltransferases including ST6GalNAc1 and ST6Gal1 alters mucus architecture, forming a thick, rigid, and disorganized layer that undermines mucosal defense[58-60]. This abnormal glycosylation promotes immune evasion through Siglec receptor engagement and facilitates tumor cell detachment and metastasis [Figure 1]. The overexpression of tumor-associated glycans such as STn and sLea, and sLex disrupts glycan homeostasis and aggravates host-microbiome imbalance. In IBD, mutations in ST6GalNAc1 have been linked to impaired sialylation, defective mucus barriers, microbial dysbiosis, and inflammation[50], underscoring its dual role in epithelial defense and protection against mucin-degrading microbes[50-56] [Figure 1].
These glycomic alterations may also reshape the microbiota [Figure 1]. CRC-associated bacteria, including Fusobacterium nucleatum, Solobacterium moorei, Atopobium parvulum, and Actinomyces odontolyticus, thrive in the altered mucosal environment[61,62] [Figure 1]. F. nucleatum expresses the adhesin Fap2, which binds tumor-associated glycans to enhance colonization and foster a pro-inflammatory, tumorigenic microenvironment[62]. However, the precise molecular interactions underlying this process remain unclear. Notably, dysbiosis in CRC is also accompanied by the production of microbial metabolites and inflammatory signals that drive genotoxic stress and activate oncogenic pathways[58,59] [Figure 1]. Recent work by Duizer et al. advanced understanding by showing that the F. nucleatum metabolite ADP-heptose activates the ALPK1-NF-κB signaling axis, inducing PD-L1 expression in CRC cells via innate immune sensing, independent of receptor-mediated contact[63]. While this reveals a metabolite-driven mechanism of immune modulation, how F. nucleatum physically engages host cells remains unresolved. Prior studies suggested that Fap2 binds Gal-GalNAc structures on tumor cells[64], but these findings rely largely on lectin-blocking assays and loss-of-function experiments, and lack definitive evidence of high-affinity glycan-protein interactions. Thus, although this work lays the foundation for targeting microbe-driven immunomodulation in cancer, further research is needed to clarify the role of glycan-mediated binding in shaping immune responses and tumor progression.
Although mucin degradation supports epithelial turnover and nutrient recycling, hypersialylated mucins preferentially sustain sialic acid-scavenging bacteria such as Escherichia coli and Akkermansia muciniphila [Figure 1]. Additional taxa - including Bacteroides fragilis, B. thetaiotaomicron, B. caccae, Ruminococcus gnavus, and Salmonella spp. - express glycosidases that directly remodel mucin glycans, further shaping microbial community composition and metabolic outputs[53].
Dietary inputs can also modulate mucin sialylation. For instance, sialylated milk oligosaccharides promote beneficial microbiota and support mucosal barrier function, linking nutrition directly to host glycan regulation[57].
In summary, the regulation of sialic acid metabolism - through host enzymes, microbial activity, and diet - plays a central role in orchestrating the glycan landscape of the gut. Both desialylation and hypersialylation represent pathogenic disruptions of glycan homeostasis, driving the breakdown of host-microbiome symbiosis and contributing to inflammatory and neoplastic diseases. Unravelling these complex interactions is crucial for developing therapeutic strategies to restore or modulate sialylation, offering promising avenues for the treatment of both IBD and CRC.
IMPLICATIONS FOR DIAGNOSIS AND THERAPY
Glycosylation changes are hallmarks of cancer and serve as valuable biomarkers for early detection and disease monitoring[38]. Advances in glycomics and analytical techniques have identified glycan-based signatures in CRC that can distinguish pathological states and predict disease progression[45]. Altered sialylation patterns, such as STn and sLex/a, have already been used as clinical biomarkers, with potential applications in non-invasive liquid biopsies through glycoproteomic profiling of circulating tumor cells or even in fecal samples[65,66]. Furthermore, these glycans may in the future serve as targets for glycan-directed molecular imaging agents to improve cancer visualization[67].
From a therapeutic perspective, one of the earliest and most notable efforts to target aberrant glycosylation involved vaccine development. For example, Theratope, a synthetic STn-based cancer vaccine designed to elicit immune responses against STn-expressing tumor cells, showed promise in early-phase trials for metastatic breast cancer[68]. However, the Phase III trial did not meet its primary endpoints[69]. Despite this, it provided valuable insights into the immunogenicity of glycan antigens and highlighted the importance of patient stratification and biomarker-driven strategies in glycan-targeted immunotherapy.
Other therapeutic strategies have focused on exploiting aberrant glycosylation with high-affinity antibodies. Over the past decades, there has been a significant increase in the number of anti-glycan antibodies and their applications[70]. Notably, a new antibody has demonstrated potential in a preclinical model as an efficient chimeric antigen receptor (CAR) against STn-positive tumors[71,72]. In addition, antibody-drug conjugates (ADCs) targeting STn have entered clinical trials (NCT04665921), reflecting both clinical and economic interest[73]. However, the trial was recently terminated due to portfolio prioritization by the sponsor (Seagen Inc.), rather than safety concerns or lack of efficacy. These experiences with vaccines and antibodies highlight both the strong scientific rationale and the challenges of bringing glycan-targeted therapies into clinical practice. Their discontinuation highlights the need for competitive positioning of glycan biomarkers and deeper mechanistic insights into glycan biology.
Immunotherapy approaches that counteract glycan-mediated immune evasion, such as Siglec-blockade therapies, are also being investigated to enhance antitumor immunity[74,75]. Furthermore, glycomimetic inhibitors of glycosyltransferases have shown promise in preclinical studies for modulating aberrant glycosylation in cancer cells[76].
Despite encouraging progress, significant challenges remain in translating glycan-based discoveries into clinical practice. Some glycan structures are shared between malignant and normal tissues, as in the case of sLex, raising concerns about unintended targeting of healthy cells and off-tumor toxicity. This narrows the therapeutic window and necessitates highly selective strategies to ensure on-target specificity, such as exploiting tumor-specific glycoforms or glycan-decorated proteins[44], or employing bispecific approaches. Tumor glycosylation patterns also vary substantially across cancer types, disease stages, and even within individual tumors. Such heterogeneity complicates biomarker prioritization and patient stratification, making personalized glycomic profiling and highly sensitive analytical platforms essential for precision therapy. Moreover, the inherent heterogeneity of the glycome and the complexity of glycan biosynthesis necessitate further research and sophisticated techniques, such as mass spectrometry and glycan arrays, which are not yet standardized or widely accessible. Nevertheless, the growing integration of glycomics with systems biology approaches holds great promise for fully unlocking the diagnostic and therapeutic potential of glycans, paving the way for more innovative and effective interventions.
CONCLUSION
The interplay between glycans and the GI microbiota constitutes a dynamic regulatory axis that is foundational to intestinal homeostasis and strongly implicated in CRC pathogenesis. In this review, we focused on O-glycan sialylation to illustrate the context-dependent role of glycans, serving as protective factors under physiological conditions but contributing to immune evasion, inflammation, and microbial dysbiosis in malignancy. This dual behavior underscores their central role in shaping the tumor microenvironment and highlights their potential as both biomarkers and therapeutic targets.
In healthy tissues, mucin-bound sialylated glycans are critical for maintaining mucosal integrity. They shield epithelial surfaces from enzymatic degradation, modulate immune responses, and promote colonization by symbiotic microbiota. In CRC, however, tumor-driven hypersialylation and the expression of aberrant glycan structures (e.g., sialyl-Tn, sLex/a) subvert these protective mechanisms. Such alterations not only facilitate immune escape and chronic inflammation, but also attract pathogenic, including sialic acid-binding, bacteria that disrupt the mucosal barrier and enhance bacterial adhesion and colonization, thereby promoting tumor development.
To fully harness the therapeutic and diagnostic potential of glycans, future research must unravel the molecular mechanisms underlying the glycan-microbiota-immune axis in CRC. This endeavor will require the integration of state-of-the-art technologies, including: advanced mass spectrometry and glycan microarrays for high-throughput, structure-specific glycomic profiling and interaction mapping; single-cell and spatial transcriptomics to chart glycan expression and immune-microbial interactions within tumor niches; clustered, regularly interspaced, short palindromic repeats (CRISPR)-based glycoengineering and organoid-microbiome co-culture systems to dissect the functional consequences of glycan alterations; and machine learning algorithms for multi-omics data integration, interaction prediction, biomarker discovery, and patient stratification.
Strategic priorities should include identifying CRC-specific glycoforms, characterizing microbial glycan-binding proteins and enzymes that remodel tumor glycomes, and designing highly specific glycan-targeted therapies, such as engineered proteins, bispecific antibodies, glycan-drug/targeting conjugates, glycoengineered biologics, or microbiota-modulating agents.
While challenges remain, particularly glycome heterogeneity and the lack of standardized clinical assays, the convergence of glycobiology with immunology, microbiome research, and computational biology positions the field for major advances. Targeting glycosylation, especially sialylation, within its biological, microbial, and immune context offers a novel systems-level approach to early detection, therapeutic precision, and improved outcomes in metastatic CRC.
DECLARATIONS
Acknowledgments
The authors acknowledge the technical support from GLYCOToolbox (UCIBIO, NOVA-FCT) for training researchers in Glycosciences. RGS gratefully acknowledges the budget from Santander Academy and Universidad Europea de Madrid, which enabled her research stay at FCT NOVA, Lisbon. Graphic Abstract created using BioRender (www.biorender.com).
Authors’ Contributions
Contributed to the conception and design of the study, wrote the original draft: Gonzalez-Soltero R, Sharma S, Videira PA
Supervised the work: Videira PA
Contributed to critically writing sections of the manuscript: Barreira DF, Benavente AL, Palma AS
Created the image of this manuscript: Barreira DF
All authors contributed to the manuscript revision, read, and approved the submitted version.
Availability of data and materials
Not applicable.
Financial support and sponsorship
This work was financed by national funds from FCT - Fundação para a Ciência e a Technologia, I.P., project UIDP/04378/2020 and UIDB/04378/2020 of the Research Unit on Applied Molecular Biosciences - UCIBIO, project LA/P/0140/2020 of the Associate Laboratory Institute for Health and Bioeconomy - i4HB and doctoral fellowships 2023.03622.BD. Additional support was provided by the European Commission through project GLYCOTwinning (GA 101079417) and project Car T Matters (n.º 18427), COMPETE2030-FEDER-01477200.
Conflicts of interest
Videira PA is an Editorial Board member of Journal of Cancer Metastasis and Treatment. She was not involved in any steps of editorial processing, notably including reviewer selection, manuscript handling, or decision making, while the other authors have declared that they have no conflicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
© The Author(s) 2025.
REFERENCES
1. Varki A. Nothing in glycobiology makes sense, except in the light of evolution. Cell. 2006;126:841-5.
2. Määttänen P, Gehring K, Bergeron JJ, Thomas DY. Protein quality control in the ER: the recognition of misfolded proteins. Semin Cell Dev Biol. 2010;21:500-11.
3. Reily C, Stewart TJ, Renfrow MB, Novak J. Glycosylation in health and disease. Nat Rev Nephrol. 2019;15:346-66.
4. Xu Y, Uddin N, Wagner GK. Chapter Nine - Covalent probes for carbohydrate-active enzymes: from glycosidases to glycosyltransferases. Methods Enzymol. 2018;598:237-65.
7. Bangarh R, Khatana C, Kaur S, et al. Aberrant protein glycosylation: implications on diagnosis and Immunotherapy. Biotechnol Adv. 2023;66:108149.
8. Purushothaman A, Mohajeri M, Lele TP. The role of glycans in the mechanobiology of cancer. J Biol Chem. 2023;299:102935.
9. La Rosa SL, Ostrowski MP, Vera-Ponce de León A, et al. Glycan processing in gut microbiomes. Curr Opin Microbiol. 2022;67:102143.
10. Verhelst X, Dias AM, Colombel JF, et al. Protein glycosylation as a diagnostic and prognostic marker of chronic inflammatory gastrointestinal and liver diseases. Gastroenterology. 2020;158:95-110.
11. Silva MC, Fernandes Â, Oliveira M, et al. Glycans as immune checkpoints: removal of branched N-glycans enhances immune recognition preventing cancer progression. Cancer Immunol Res. 2020;8:1407-25.
12. Xu X, Peng Q, Jiang X, et al. Altered glycosylation in cancer: molecular functions and therapeutic potential. Cancer Commun. 2024;44:1316-36.
13. Fernandes A, Azevedo CM, Silva MC, et al. Glycans as shapers of tumour microenvironment: a sweet driver of T-cell-mediated anti-tumour immune response. Immunology. 2023;168:217-32.
14. Crocker PR, Gordon S. Properties and distribution of a lectin-like hemagglutinin differentially expressed by murine stromal tissue macrophages. J Exp Med. 1986;164:1862-5.
15. Angata T, Tabuchi Y, Nakamura K, Nakamura M. Siglec-15: an immune system Siglec conserved throughout vertebrate evolution. Glycobiology. 2007;17:838-6.
16. Lehmann F, Gäthje H, Kelm S, Dietz F. Evolution of sialic acid-binding proteins: Molecular cloning and expression of fish siglec-4. Glycobiology. 2004;14:959-8.
17. Angata T, Margulies EH, Green ED, Varki A. Large-scale sequencing of the CD33-related Siglec gene cluster in five mammalian species reveals rapid evolution by multiple mechanisms. Proc Natl Acad Sci USA. 2004;101:13251-6.
18. Duan S, Paulson JC. Siglecs as immune cell checkpoints in disease. Annu Rev Immunol. 2020;38:365-95.
19. Pillai S, Netravali IA, Cariappa A, Mattoo H. Siglecs and immune regulation. Annu Rev Immunol. 2012;30:357-92.
20. Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol. 2007;7:255-66.
21. Macauley MS, Crocker PR, Paulson JC. Siglec-mediated regulation of immune cell function in disease. Nat Rev Immunol. 2014;14:653-66.
22. Hudak JE, Canham SM, Bertozzi CR. Glycocalyx engineering reveals a Siglec-based mechanism for NK cell immunoevasion. Nat Chem Biol. 2014;10:69-75.
23. Santegoets KCM, Gielen PR, Büll C, et al. Expression profiling of immune inhibitory Siglecs and their ligands in patients with glioma. Cancer Immunol Immunother. 2019;68:937-49.
24. Silva Z, Rabaça JA, Luz V, et al. New insights into the immunomodulatory potential of sialic acid on monocyte-derived dendritic cells. Cancer Immunol Immunother. 2024;74:9.
25. Silva Z, Soares CO, Barbosa M, Palma AS, Marcelo F, Videira PA. The role of sialoglycans in modulating dendritic cell function and tumour immunity. Semin Immunol. 2024;74-5:101900.
26. Edwards DL, Huang M, Wang TT. Soluble factors and mechanisms regulated by sialylated IgG signaling. Immunol Rev. 2025;330:e70021.
27. Dall'Olio F, Malagolini N, Trinchera M, Chiricolo M. Sialosignaling: sialyltransferases as engines of self-fueling loops in cancer progression. Biochim Biophys Acta. 2014;1840:2752-64.
28. Harduin-lepers. Comprehensive analysis of sialyltransferases in vertebrate genomes. Glycobiol Insights. 2010;2:29-61.
29. Picco G, Julien S, Brockhausen I, et al. Over-expression of ST3Gal-I promotes mammary tumorigenesis. Glycobiology. 2010;20:1241-50.
30. Kvorjak M, Ahmed Y, Miller ML, et al. Cross-talk between colon cells and macrophages increases ST6GALNAC1 and MUC1-sTn expression in ulcerative colitis and colitis-associated colon cancer. Cancer Immunol Res. 2020;8:167-78.
31. Ferreira JA, Videira PA, Lima L, et al. Overexpression of tumour-associated carbohydrate antigen sialyl-Tn in advanced bladder tumours. Mol Oncol. 2013;7:719-31.
32. Carrascal MA, Severino PF, Guadalupe Cabral M, et al. Sialyl Tn-expressing bladder cancer cells induce a tolerogenic phenotype in innate and adaptive immune cells. Mol Oncol. 2014;8:753-65.
33. Jiang Y, Liu Z, Xu F, et al. Aberrant O-glycosylation contributes to tumorigenesis in human colorectal cancer. J Cell Mol Med. 2018;22:4875-85.
34. Zeng J, Mi R, Wang Y, et al. Promoters of human cosmc and T-synthase genes are similar in structure, yet different in epigenetic regulation. J Biol Chem. 2015;290:19018-33.
35. Sun X, Ju T, Cummings RD. Differential expression of Cosmc, T-synthase and mucins in Tn-positive colorectal cancers. BMC Cancer. 2018;18:1-15.
36. Gao T, Du T, Hu X, et al. Cosmc overexpression enhances malignancies in human colon cancer. J Cell Mol Med. 2020;24:362-70.
37. Futamura N, Nakamura S, Tatematsu M, Yamamura Y, Kannagi R, Hirose H. Clinicopathologic significance of sialyl Le xexpression in advanced gastric carcinoma. Br J Cancer. 2000;83:1681-7.
38. Shen L, Luo Z, Wu J, et al. Enhanced expression of α2,3-linked sialic acids promotes gastric cancer cell metastasis and correlates with poor prognosis. Int J Oncol. 2017;50:1201-10.
39. Zhou X, Chi K, Zhang C, Liu Q, Yang G. Sialylation: a cloak for tumors to trick the immune system in the microenvironment. Biology. 2023;12:832.
40. Venkitachalam S, Revoredo L, Varadan V, et al. Biochemical and functional characterization of glycosylation-associated mutational landscapes in colon cancer. Sci Rep. 2016;6:23642.
41. Wang D, Kuzyk V, Madunić K, et al. In-depth analysis of the N-glycome of colorectal cancer cell lines. Int J Mol Sci. 2023;24:4842.
42. Rodrigues JG, Duarte HO, Gomes C, et al. Terminal α2,6-sialylation of epidermal growth factor receptor modulates antibody therapy response of colorectal cancer cells. Cell Oncol. 2021;44:835-50.
43. Mereiter S, Balmaña M, Gomes J, Magalhães A, Reis CA. Glycomic approaches for the discovery of targets in gastrointestinal cancer. Front Oncol. 2016;6:55.
44. Deschepper FM, Zoppi R, Pirro M, et al. L1CAM as an E-selectin ligand in colon cancer. Int J Mol Sci. 2020;21:8286.
45. Wang D, Madunić K, Mayboroda OA, Lageveen-Kammeijer GSM, Wuhrer M. (Sialyl)Lewis antigen expression on glycosphingolipids, N-, and O-glycans in colorectal cancer cell lines is linked to a colon-like differentiation program. Mol Cell Proteomics. 2024;23:100776.
46. Gomes C, Osório H, Pinto MT, Campos D, Oliveira MJ, Reis CA. Expression of ST3GAL4 leads to SLe xexpression and induces c-Met activation and an invasive phenotype in gastric carcinoma cells. PLoS One. 2013;8:e66737.
47. Tian H, Yu JL, Chu X, Guan Q, Liu J, Liu Y. Unraveling the role of C1GALT1 in abnormal glycosylation and colorectal cancer progression. Front Oncol. 2024;14:1389713.
48. Vázquez-Martín C, Cuevas E, Gil-Martín E, Fernández-Briera A. Correlation analysis between tumor-associated antigen sialyl-Tn expression and ST6GalNAc I activity in human colon adenocarcinoma. Oncology. 2004;67:159-65.
49. Dombek GE, Ore AS, Cheng J, et al. Immunohistochemical analysis of Tn antigen expression in colorectal adenocarcinoma and precursor lesions. BMC Cancer. 2022;22:1281.
50. Yao Y, Kim G, Shafer S, et al. Mucus sialylation determines intestinal host-commensal homeostasis. Cell. 2022;185:1172-88.e28.
51. Lee S, Inzerillo S, Lee GY, Bosire EM, Mahato SK, Song J. Glycan-mediated molecular interactions in bacterial pathogenesis. Trends Microbiol. 2022;30:254-67.
52. Kotlarz D. Mucus sialylation maintains the peace in intestinal host microbe relations. Gastroenterology. 2022;163:527-8.
53. Taniguchi M, Okumura R, Matsuzaki T, et al. Sialylation shapes mucus architecture inhibiting bacterial invasion in the colon. Mucosal Immunol. 2023;16:624-41.
54. Dedola S, Ahmadipour S, de Andrade P, et al. Sialic acids in infection and their potential use in detection and protection against pathogens. RSC Chem Biol. 2024;5:167-88.
55. Arai J, Hayakawa Y, Tateno H, Fujiwara H, Kasuga M, Fujishiro M. The role of gastric mucins and mucin-related glycans in gastric cancers. Cancer Sci. 2024;115:2853-61.
56. Leite-Gomes E, Dias AM, Azevedo CM, et al. Bringing to light the risk of colorectal cancer in inflammatory bowel disease: mucosal glycosylation as a key player. Inflamm Bowel Dis. 2022;28:947-62.
57. Okumura R, Takeda K. The role of the mucosal barrier system in maintaining gut symbiosis to prevent intestinal inflammation. Semin Immunopathol. 2024;47:2.
58. Pothuraju R, Rachagani S, Krishn SR, et al. Molecular implications of MUC5AC-CD44 axis in colorectal cancer progression and chemoresistance. Mol Cancer. 2020;19:37.
60. Ma X, Li M, Wang X, Qi G, Wei L, Zhang D. Sialylation in the gut: from mucosal protection to disease pathogenesis. Carbohydr Polym. 2024;343:122471.
61. Wong SH, Yu J. Gut microbiota in colorectal cancer: mechanisms of action and clinical applications. Nat Rev Gastroenterol Hepatol. 2019;16:690-704.
62. Casasanta MA, Yoo CC, Udayasuryan B, et al. Fusobacterium nucleatum host-cell binding and invasion induces IL-8 and CXCL1 secretion that drives colorectal cancer cell migration. Sci Signal. 2020;13:eaba9157.
63. Duizer C, Salomons M, van Gogh M, et al. Fusobacterium nucleatum upregulates the immune inhibitory receptor PD-L1 in colorectal cancer cells via the activation of ALPK1. Gut Microbes. 2025;17:2458203.
64. Abed J, Emgård JE, Zamir G, et al. Fap2 mediates fusobacterium nucleatum colorectal adenocarcinoma enrichment by binding to tumor-expressed Gal-GalNAc. Cell Host Microbe. 2016;20:215-25.
65. Caldevilla R, Eiras M, Santos DAR, et al. Advancing non-invasive colorectal cancer screening: exploring the potential of monoclonal antibody L2A5. Int J Mol Sci. 2025;26:3070.
66. Neves M, Azevedo R, Lima L, et al. Exploring sialyl-Tn expression in microfluidic-isolated circulating tumour cells: a novel biomarker and an analytical tool for precision oncology applications. N Biotechnol. 2019;49:77-87.
67. Houvast RD, Vankemmelbeke M, Durrant LG, et al. Targeting glycans and heavily glycosylated proteins for tumor imaging. Cancers. 2020;12:3870.
68. Ibrahim NK, Murray JL. Clinical development of the STn-KLH vaccine (Theratope). Clin Breast Cancer. 2003;3 Suppl 4:S139-43.
69. Holmberg LA, Sandmaier BM. Vaccination with Theratope® (STn-KLH) as treatment for breast cancer. Expert Rev Vaccines. 2004;3:655-63.
70. Sterner E, Flanagan N, Gildersleeve JC. Perspectives on anti-glycan antibodies gleaned from development of a community resource database. ACS Chem Biol. 2016;11:1773-83.
71. Loureiro LR, Feldmann A, Bergmann R, et al. Extended half-life target module for sustainable UniCAR T-cell treatment of STn-expressing cancers. J Exper Clin Cancer Res. 2020;39:77.
72. Loureiro LR, Feldmann A, Bergmann R, et al. Development of a novel target module redirecting UniCAR T cells to Sialyl Tn-expressing tumor cells. Blood Cancer J. 2018;8:81.
73. Al-Alem L, Prendergast JM, Clark J, et al. Sialyl-Tn serves as a potential therapeutic target for ovarian cancer. J Ovarian Res. 2024;17:71.
74. Manni M, Läubli H. Targeting glyco-immune checkpoints for cancer therapy. Expert Opin Biol Ther. 2021;21:1063-71.
75. Videira PA, Marcelo F, Grewal RK. Glycosyltransferase inhibitors: a promising strategy to pave a path from laboratory to therapy. Carbohydr Chem. 2017;43:135-58.
Cite This Article
How to Cite
Download Citation
Export Citation File:
Type of Import
Tips on Downloading Citation
Citation Manager File Format
Type of Import
Direct Import: When the Direct Import option is selected (the default state), a dialogue box will give you the option to Save or Open the downloaded citation data. Choosing Open will either launch your citation manager or give you a choice of applications with which to use the metadata. The Save option saves the file locally for later use.
Indirect Import: When the Indirect Import option is selected, the metadata is displayed and may be copied and pasted as needed.
About This Article
Copyright
Data & Comments
Data

















Comments
Comments must be written in English. Spam, offensive content, impersonation, and private information will not be permitted. If any comment is reported and identified as inappropriate content by OAE staff, the comment will be removed without notice. If you have any queries or need any help, please contact us at support@oaepublish.com.